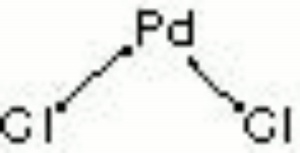Difference between revisions of "Palladium chloride"
Jump to navigation
Jump to search
(username removed) |
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
||
| Line 41: | Line 41: | ||
R. Waller, K.Andrew, J.Tetreault, "Survey of Gaseous Pollutant Concentration Distributions in Mineral Collections" Collection Forum, 14(1-2):1-32, 2000. | R. Waller, K.Andrew, J.Tetreault, "Survey of Gaseous Pollutant Concentration Distributions in Mineral Collections" Collection Forum, 14(1-2):1-32, 2000. | ||
| − | == | + | == Sources Checked for Data in Record == |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Revision as of 13:31, 1 May 2016
Description
Dark brown deliquescent powder that is used as a brightener in metal coatings. Palladium chloride is also used in indelible inks, photography, and glassmaking. Solutions of palladium chloride have been used to make indicator papers that are sensitive to mercury vapors (Waller et al 2000).
Synonyms and Related Terms
palladous chloride; palladium dichloride; palladium (II) chloride
Other Properties
Soluble in water, hydrochloric acid, ethanol, acetone.
| Composition | PdCl2 |
|---|---|
| CAS | 7647-10-1 |
| Melting Point | 675 |
| Density | 4.0 |
| Molecular Weight | mol. wt. = 177.306 |
Hazards and Safety
Suspected carcinogen. Hygroscopic. Contact may cause irritation.
Fisher Scientific: MSDS
Additional Information
R. Waller, K.Andrew, J.Tetreault, "Survey of Gaseous Pollutant Concentration Distributions in Mineral Collections" Collection Forum, 14(1-2):1-32, 2000.
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979