Difference between revisions of "Propionic acid"
Jump to navigation
Jump to search
(username removed) |
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
||
| Line 43: | Line 43: | ||
Mallinckrodt Baker: [http://www.jtbaker.com/msds/englishhtml/p6643.htm MSDS] | Mallinckrodt Baker: [http://www.jtbaker.com/msds/englishhtml/p6643.htm MSDS] | ||
| − | == | + | == Sources Checked for Data in Record == |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 9 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 9 | ||
Revision as of 14:15, 1 May 2016
Description
Colorless oily liquid with a irritating, rancid smell. Propionic acid occurs naturally in small amounts in dairy products and wood pulp. It is used as a mold inhibitor and preservative.
Synonyms and Related Terms
methylacetic acid; propanoic acid; ethylformic acid
Other Properties
Soluble in water, ethanol, chloroform, ether.
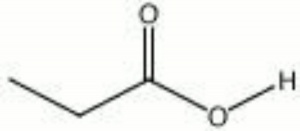
| Composition | CH3CH2CO2H |
|---|---|
| CAS | 79-09-4 |
| Melting Point | -20.8 |
| Density | 0.99 |
| Molecular Weight | mol. wt. = 74.08 |
| Refractive Index | 1.3862 |
| Boiling Point | 140.7 |
Hazards and Safety
Flammable. Flash point = 52 C. Corrosive. Causes burns on contact. Harmful in inhalation, ingestion and skin absorption.
Mallinckrodt Baker: MSDS
Sources Checked for Data in Record
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 9
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: ref. index=1.3862
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.385