Difference between revisions of "Sodium acetate"
Jump to navigation
Jump to search
(username removed) |
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
||
| Line 40: | Line 40: | ||
LINK: [http://www.cdc.gov/niosh/ipcsneng/neng0565.html International Chemical Safety Card] | LINK: [http://www.cdc.gov/niosh/ipcsneng/neng0565.html International Chemical Safety Card] | ||
| − | == | + | == Sources Checked for Data in Record == |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Revision as of 18:36, 1 May 2016
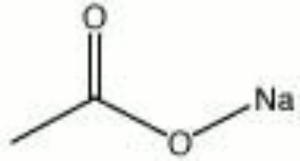
Description
A colorless, hygroscopic powder. Hydrated sodium acetate is used in photographic emulsions as a buffer. It is also used as a mordant for dyeing textiles and as a preservative in tanning hides.
Synonyms and Related Terms
sodium acetate trihydrate; Natriumacetat (Deut.); acetato de sódio (Port.)
Other Properties
Soluble in water. Slightly soluble in ethanol. Soluble in ether.
| Composition | NaC2H3O2 |
|---|---|
| CAS | 127-09-3 (anhydrous) |
| Melting Point | 58 |
| Density | 1.45 |
| Molecular Weight | mol. wt. = 82.04 |
| Boiling Point | 324 |
Hazards and Safety
Combustible. Contact may cause irritation.
LINK: International Chemical Safety Card
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8711
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Sodium_acetate (Jan. 6 2006)