Difference between revisions of "Trichloroethane, 1,1,2-"
Jump to navigation
Jump to search
(username removed) |
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
||
| Line 45: | Line 45: | ||
LINK: [http://www.cdc.gov/niosh/ipcsneng/neng0080.html International Chemical Safety Card] | LINK: [http://www.cdc.gov/niosh/ipcsneng/neng0080.html International Chemical Safety Card] | ||
| − | == | + | == Sources Checked for Data in Record == |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Revision as of 21:26, 1 May 2016
Description
Colorless, sweet-smelling liquid. 1,1,2-trichloroethane is used as a solvent for fats, oils, waxes, resins, and polymers.
Synonyms and Related Terms
vinyl trichloride; beta-chloroethane; beta-trichloroethane
Other Properties
Soluble in ethanol, ethers, esters, ketones. Insoluble in water.
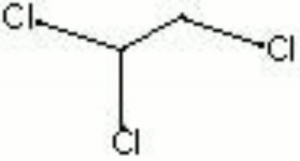
| Composition | CHCl2CH2Cl |
|---|---|
| CAS | 79-00-5 |
| Melting Point | -36.4 |
| Density | 1.4432 |
| Molecular Weight | mol. wt.=133.4 |
| Refractive Index | 1.4458-1.4711 |
| Boiling Point | 113.7 |
Hazards and Safety
Nonflammable but decomposed with heat to produce toxic fumes.
Skin contact causes irritation. Potential carcinogen. Inhalation or ingestion may cause nausea, heart arrhythmia, headaches, liver damage.
LINK: International Chemical Safety Card
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9767