Difference between revisions of "Triphenyltetrazolium chloride"
Jump to navigation
Jump to search
(username removed) |
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
||
| Line 36: | Line 36: | ||
Fisher Scientific: [https://fscimage.fishersci.com/msds/01820.htm MSDS] | Fisher Scientific: [https://fscimage.fishersci.com/msds/01820.htm MSDS] | ||
| − | == | + | == Sources Checked for Data in Record == |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Revision as of 21:28, 1 May 2016
Description
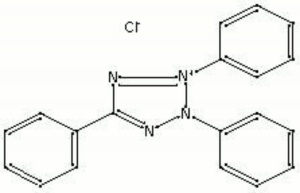
Colorless needle crystals that yellow when exposed to light. Triphenyltetrazolium chloride (TTC) is primarily used as an analytical reagent for the detection of reducing sugars in plant and animal tissues. The colorless TTC reacts with sugars to become a deep red, water-insoluble compound (triphenylformazan). Reducing agents (i.e. borohydride, bleaching powder, chloramine T) give a positive red result.
Synonyms and Related Terms
TTC; TPTZ; red tetrazolium; tetrazolium chloride; 2,3,5-triphenyl-2H-tetrazolium chloride; Vitastain; tetrazole red
Other Properties
Soluble in ethanol, water, acetone. Insoluble in ether.
| Composition | C19H15ClN4 |
|---|---|
| CAS | 298-96-4 |
| Melting Point | 243 (dec) |
| Molecular Weight | mol. wt. = 334.5848 |
Hazards and Safety
Flammable. Flash point = 40C
Contact causes irritation.
Fisher Scientific: MSDS
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, Notes for Workshop on New Methods in the Cleaning of Paintings, J.Paul Getty Trust, Los Angeles, 1990
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9874
- Aldrich Chemical Catalog Comment: p. 1502