Difference between revisions of "Cobalt oxide"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 16: | Line 16: | ||
Insoluble in water | Insoluble in water | ||
| − | == | + | == Sources Checked for Data in Record == |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Revision as of 13:44, 29 April 2016
Description
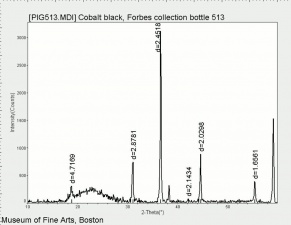
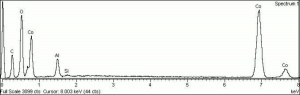
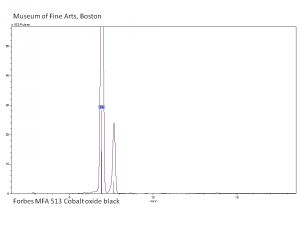
A natural mixture of two or more cobalt oxides. The most common forms of cobalt oxide are: Cobaltous oxide (CoO), Cobaltic oxide (Co2O3), and tricobalt tetroxide (or cobalto-cobaltic oxide, Co3O4). The latter contains cobalt in both valences and comprises up to 40 percent of the commercial cobalt oxide used in the manufacture of ceramics, glass, and enamel and in the preparation of catalysts and cobalt metal powder. Cobalt oxide was used as a blue colorant in ceramic glazes and underglazes since at least the 8th century in the Middle East. It became very popular in the 14th century when it was used for Ming Dynasty ceramics.
Synonyms and Related Terms
oxyde de cobalt (Fr.); óxido de cobalto (Esp., Port.); osido di cobalto (It.)
Examples include: cobaltous oxide; cobaltic oxide; tricobalt tetroxide; cobalto-cobaltic oxide
Other Properties
Insoluble in water
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Encyclopedia Britannica, http://www.britannica.com Comment: "cobalt processing." Encyclopædia Britannica. 2005. Encyclopædia Britannica Premium Service 17 Mar. 2005 .
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Cobalt_oxide (Accessed Jan. 15, 2006)
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000