Difference between revisions of "Dinitrophenol"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 39: | Line 39: | ||
[http://www.cdc.gov/niosh/ipcsneng/neng0464.html International Chemical Safety Card] | [http://www.cdc.gov/niosh/ipcsneng/neng0464.html International Chemical Safety Card] | ||
| − | == | + | == Sources Checked for Data in Record == |
* ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 | ||
Revision as of 19:47, 30 April 2016
Description
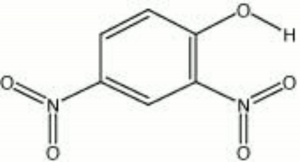
A yellow crystalline material used in the manufacture of sulfur dyes and photographic developer. Dinitrophenol is a chromotropic pH indicator that is colorless below pH 2.6 and yellow above pH 4.4. It is also used as a wood preservative, an Insecticide, and a reagent for the detection of Potassium and ammonium ions.
Synonyms and Related Terms
2,4-dinitrophenol; alpha-dinitrophenol; dinitrophenol (Fr.); Aldifen
Other Properties
Soluble in ethanol, ether, benzene and chloroform. Slightly soluble in water.
| Composition | C6H3OH(NO2)2 |
|---|---|
| CAS | 51-28-5 |
| Melting Point | 112-114 |
| Density | 1.683 |
| Molecular Weight | mol. wt.=184.11 |
Hazards and Safety
Combustible. Explosion hazard when dry.
Highly toxic by skin absorption, ingestion and inhalation.
International Chemical Safety Card
Sources Checked for Data in Record
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993