Difference between revisions of "Gold trichloride"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 37: | Line 37: | ||
Fisher Scientific: [https://fscimage.fishersci.com/msds/99144.htm MSDS] | Fisher Scientific: [https://fscimage.fishersci.com/msds/99144.htm MSDS] | ||
| − | == | + | == Sources Checked for Data in Record == |
* Thomas Gregory, ''The Condensed Chemical Dictionary'', Reinhold Publishing, New York, 3rd ed., 1942 | * Thomas Gregory, ''The Condensed Chemical Dictionary'', Reinhold Publishing, New York, 3rd ed., 1942 | ||
Revision as of 21:11, 30 April 2016
Description
Dark orange crystals that decompose with light or heat. An aqueous solution is called chlorauric acid or acid gold trichloride. Gold trichloride is used as a toner for black and white photographs. It is also used as a glaze for ceramics, enameling glass and making Ruby glass. Other uses of gold trichloride include gold plating and the production of fine gold powder.
Synonyms and Related Terms
auric chloride; auric trichloride; gold (III) chloride
Other Properties
Soluble in water, ethanol and ether.
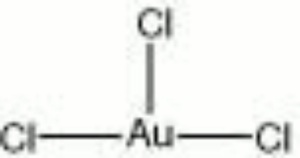
| Composition | AuCl3 |
|---|---|
| CAS | 13453-07-1 |
| Density | 3.9 |
| Molecular Weight | mol. wt. = 303.32 |
| Boiling Point | 229 |
Hazards and Safety
Decomposes with heat. Very hygroscopic. Contact, inhalation, and ingestion cause irritation and blisters. May cause severe allergic reactions.
Fisher Scientific: MSDS
Sources Checked for Data in Record
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4542
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979