Difference between revisions of "Lapis lazuli"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 60: | Line 60: | ||
| − | == | + | == Sources Checked for Data in Record == |
* Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | * Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | ||
Revision as of 06:13, 1 May 2016
Description
A brilliant azure-blue color gemstone. Lapis lazuli is a mixture of minerals, primarily containing the Lazurite (blue) with small amounts of Calcite, Sodalite, and gold-color flecks of Pyrite. Lazurite is a sodium, calcium, aluminum sulfo-chlorosilicate. Lapis lazuli has been commercially mined since 3100 BCE at the Badakhshan mines in Afghanistan. Other mines are found in Argentina, Siberia (Lake Baikal), Chile, Myanmar (formerly Burma), Pakistan, and the U.S. (California). The semiprecious blue stone was, and still is, used for jewelry, mosaics and small carvings. Lapis lazuli was also ground and purified to make natural ultramarine blue pigments. Many current pieces of lapis on the market have been dyed or waxed to improve their appearance.
Synonyms and Related Terms
lazurite; lapis; Persian blue; Fra Angelico Blue; Armenian stone; blue stone; ultramarine blue (pigment); Lapislazuli, Ultramarin (Deut.); lapislázuli (Esp.); lapis lázuli (Esp.); lapis-lazuli (Fr.); lazuryt (Pol.); lápis-lazuli (Port.); lapis lazuli (Ned.);
Other Properties
Decomposed by hydrochloric acid with the precipitation of silica, evolution of hydrogen sulfide (rotten egg smell), and effervescence from calcite inclusions.
Isometric with compact masses. Poor cleavage. Fracture = uneven. Luster = dull to greasy. Streak = pale blue.
Dyes may often be detected with an acetone swab.
| Composition | (Na,Ca)4(Al,SiO4)3(SO4,S,Cl) |
|---|---|
| Mohs Hardness | 5.0 - 5.5 |
| Density | 2.7-2.9 |
| Refractive Index | 1.50-1.67 |
Hazards and Safety
Ingestion may result in poisoning if stomach acid is high. Inhalation and contact may irritate tissue.
Additional Information
° Mineralogy Database: Lazurite ° Michael O'Donoghue and Louise Joyner, Identification of Gemstones, Butterworth-Heinemann, Oxford, 2003
Comparisons
Properties of Common Gemstones
Characteristics of Common Blue Pigments
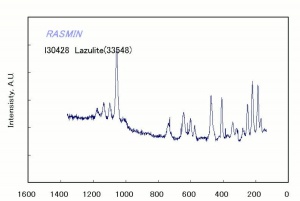
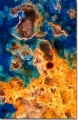
Additional Images
Sources Checked for Data in Record
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- Encyclopedia of Archaeology, Glyn E. Daniel, ed., Thomas Y. Crowell Co., New York, 1977
- R.F.Symmes, T.T.Harding, Paul Taylor, Rocks, Fossils and Gems, DK Publishing, Inc., New York City, 1997
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Lapis_lazuli (Accessed Nov. 9, 2005)
- Yasukazu Suwa, Gemstones: Quality and Value, Volume 1, Sekai Bunka Publishing Inc., Tokyo, 1999 Comment: RI=1.50-1.67; Specific gravity=2.75
- Michael O'Donoghue and Louise Joyner, Identification of Gemstones, Butterworth-Heinemann, Oxford, 2003 Comment: RI=1.50; Specific gravity=2.7-2.9
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 611
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979