Difference between revisions of "Lead tetroxide"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 44: | Line 44: | ||
E. West-Fitzhugh, "Red Lead and Minium", ''Artists Pigments'', Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986. | E. West-Fitzhugh, "Red Lead and Minium", ''Artists Pigments'', Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986. | ||
| − | == | + | == Sources Checked for Data in Record == |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 8.73 and ref. index = 2.42 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 8.73 and ref. index = 2.42 | ||
Revision as of 06:22, 1 May 2016
Description
A heavy, bright red powder that occurs naturally as the mineral Minium. The mineral form, however, was rarely used. Instead lead tetroxide was prepared by slowly heating lead monoxide in a furnace with a constant flow of air. Excess heating will turn the pigment to an orange shade that is known as Orange mineral. Lead tetroxide, also called Red lead oxide, was one of the earliest synthetic pigments. It is no longer used as an artists color because is has poor light stability and poor working properties. Instead, red lead is used to color glass, enamels, and ceramic glazes. It is also used as a flux in porcelain painting, an anticorrosive paint for iron and steel, and as a component in lead batteries. Because of its toxicity, the use of red lead is declining.
Synonyms and Related Terms
red lead; Pigment Red 105; CI 77578; mønje (Dan.); minio (Esp.); tetróxido de chumbo (Port.); minium (Fr.); lead oxide red; lead tetraoxide; orange mineral; Saturn red; Paris red; minio; burnt white lead; red oxide of lead; plumbous plumbate; lead orthoplumbate; mineral red
Other Properties
Soluble in glacial acetic acid, hot HCl, nitric acid with peroxide present. Insoluble in water and ethanol.
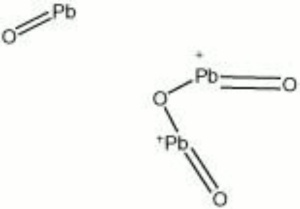
| Composition | Pb3O4 |
|---|---|
| CAS | 1314-41-6 |
| Density | 8.32-9.16 |
| Molecular Weight | mol. wt. = 685.6 |
| Refractive Index | 2.42 |
Hazards and Safety
Toxic by inhalation or ingestion. Skin contact may cause irritation or ulcers. Carcinogen, teratogen, suspected mutagen. Discolored by hydrogen sulfide.
Mallinckrodt Baker: MSDS
Additional Information
E. West-Fitzhugh, "Red Lead and Minium", Artists Pigments, Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986.
Sources Checked for Data in Record
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 8.73 and ref. index = 2.42
- Artists' Pigments: A Handbook of their History and Characteristics, R.L.Feller, ed., Cambridge University Press, London, Vol. 1, 1986 Comment: E. West-Fitzhugh, "Red Lead and Minium"
- Website address 1 Comment: Pigments Through The Ages - http://webexhibits.org/pigments/indiv/technical/redlead.html - Crystal system: tetragonal Refractive index: 2.42
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5449
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Lead_tetroxide (Accessed Feb. 2, 2006)
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979