Difference between revisions of "Ammonium dichromate"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 9: | Line 9: | ||
[[[SliderGallery rightalign|ammonium dichromate.jpg~Chemical structure]]] | [[[SliderGallery rightalign|ammonium dichromate.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Corrosive. Contact causes burns to skin and membranes. | ||
| + | * Strong Oxidizer. Contact with other materials may cause flames. | ||
| + | * Carcinogenic. Toxic by inhalation and ingestion. | ||
| + | * Fisher Scientific: [https://www.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-a/S25169B.pdf SDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in water and ethanol. | Soluble in water and ethanol. | ||
| Line 22: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 180 (dec) | + | | 180 C (dec) |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.155 | + | | 2.155 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 38: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 12:54, 26 April 2022
Description
Orange-red prismatic needles. Ammonium dichromate is mixed with Albumin and water to make a light sensitive emulsion for photolithography. It is also used in photography, process engraving, leather tanning, porcelain glazes, textile dyeing, and pyrotechnics.
Synonyms and Related Terms
ammonium bichromate
Risks
- Corrosive. Contact causes burns to skin and membranes.
- Strong Oxidizer. Contact with other materials may cause flames.
- Carcinogenic. Toxic by inhalation and ingestion.
- Fisher Scientific: SDS
Physical and Chemical Properties
Soluble in water and ethanol.
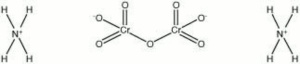
| Composition | (NH4)2Cr2O7 |
|---|---|
| CAS | 7789-09-5 |
| Melting Point | 180 C (dec) |
| Density | 2.155 g/ml |
| Molecular Weight | mol. wt. = 252.07 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 544