Difference between revisions of "Barium carbonate"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 3: | Line 3: | ||
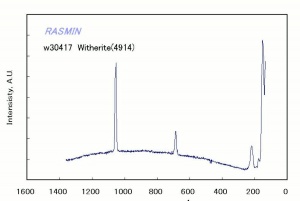
A heavy white powder that occurs in nature as the mineral [[witherite]]. Barium carbonate is used as a pigment in the manufacture of paints, glazes and synthetic marble. When [[barium hydroxide]] is used as an alkalizing agent for paper, it can react with [[carbon dioxide]] and precipitate as barium carbonate to provide an alkaline reserve. Both barium hydroxide and barium carbonate are highly toxic. Barium carbonate is used commercially in rat poison, bricks, cement, and mortar. | A heavy white powder that occurs in nature as the mineral [[witherite]]. Barium carbonate is used as a pigment in the manufacture of paints, glazes and synthetic marble. When [[barium hydroxide]] is used as an alkalizing agent for paper, it can react with [[carbon dioxide]] and precipitate as barium carbonate to provide an alkaline reserve. Both barium hydroxide and barium carbonate are highly toxic. Barium carbonate is used commercially in rat poison, bricks, cement, and mortar. | ||
| − | + | [[[SliderGallery rightalign|witheriteRS.jpg~Raman|barium carbonate.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
witherite; carbonate de baryum (Fr.); Pigment White 10; CI 77099 | witherite; carbonate de baryum (Fr.); Pigment White 10; CI 77099 | ||
| − | + | == Risks == | |
| − | + | * Highly toxic by ingestion. | |
| + | * Contact with skin and membranes may cause irritation. | ||
| + | * Integra Chem: [http://www.integrachem.com/msds/B141_25191_101.pdf SDS] | ||
| − | + | ==Physical and Chemical Properties== | |
| − | Flat tablets that are colorless under plane-polarized light; high brefringence with complete extinction; interference colors are often seen. Fluoresces a light blue color in both long and short wave UV | + | * Soluble in acid. Insoluble in water. |
| + | * Flat tablets that are colorless under plane-polarized light; high brefringence with complete extinction; interference colors are often seen. | ||
| + | * Fluoresces a light blue color in both long and short wave UV | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 28: | Line 32: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 811 | + | | 811 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 4.275 | + | | 4.275 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 40: | Line 44: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 1300 | + | | 1300 C |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 53: | Line 51: | ||
[[media:download_file_537.pdf|Characteristics of Common White Pigments]] | [[media:download_file_537.pdf|Characteristics of Common White Pigments]] | ||
| − | + | ==Resources and Citations== | |
| − | |||
| − | == | ||
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
| Line 73: | Line 69: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: Entry # 966 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: Entry # 966 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Barium_carbonate (Accessed Sept 2, 2005; hardness = 3.5, sp = 4.3) |
* Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 | * Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 | ||
Latest revision as of 09:34, 2 May 2022
Description
A heavy white powder that occurs in nature as the mineral Witherite. Barium carbonate is used as a pigment in the manufacture of paints, glazes and synthetic marble. When Barium hydroxide is used as an alkalizing agent for paper, it can react with Carbon dioxide and precipitate as barium carbonate to provide an alkaline reserve. Both barium hydroxide and barium carbonate are highly toxic. Barium carbonate is used commercially in rat poison, bricks, cement, and mortar.
Synonyms and Related Terms
witherite; carbonate de baryum (Fr.); Pigment White 10; CI 77099
Risks
- Highly toxic by ingestion.
- Contact with skin and membranes may cause irritation.
- Integra Chem: SDS
Physical and Chemical Properties
- Soluble in acid. Insoluble in water.
- Flat tablets that are colorless under plane-polarized light; high brefringence with complete extinction; interference colors are often seen.
- Fluoresces a light blue color in both long and short wave UV
| Composition | BaCO3 |
|---|---|
| CAS | 513-77-9 |
| Mohs Hardness | 3.5 |
| Melting Point | 811 C |
| Density | 4.275 g/ml |
| Molecular Weight | mol. wt. = 197.34 |
| Refractive Index | 1.529; 1.677; 1.676 |
| Boiling Point | 1300 C |
Comparisons
Characteristics of Common White Pigments
Resources and Citations
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density 4.3 and ref. index 1.529; 1.677; 1.676
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 84
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: Entry # 966
- Wikipedia: http://en.wikipedia.org/wiki/Barium_carbonate (Accessed Sept 2, 2005; hardness = 3.5, sp = 4.3)
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 Comment: transparent white pigment