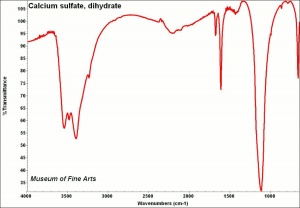
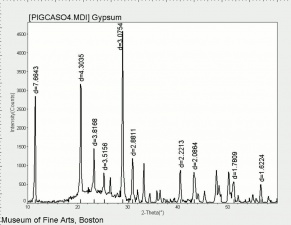
Difference between revisions of "Calcium sulfate, dihydrate"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 9: | Line 9: | ||
[[[SliderGallery rightalign|CalciumsulfatedihydrateMFAIR.jpg~FTIR|PIGCASO4.jpg~XRD]]] | [[[SliderGallery rightalign|CalciumsulfatedihydrateMFAIR.jpg~FTIR|PIGCASO4.jpg~XRD]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Noncombustible. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC446380010&productDescription=CALCIUM+SULFATE+DIHYDRAT+1KG&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Soluble in water. Slightly soluble in glycerol. Insoluble in most organic solvents. Gypsum fluoresces purple. | Soluble in water. Slightly soluble in glycerol. Insoluble in most organic solvents. Gypsum fluoresces purple. | ||
| Line 25: | Line 30: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 100-150 | + | | 100-150 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.32-2.36 | + | | 2.32-2.36 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 36: | Line 41: | ||
| 1.520; 1.530; 1.523 | | 1.520; 1.530; 1.523 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 47: | Line 46: | ||
[[media:download_file_530.pdf|Characteristics of Common White Pigments]] | [[media:download_file_530.pdf|Characteristics of Common White Pigments]] | ||
| − | + | ==Resources and Citations== | |
| − | |||
| − | == | ||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Revision as of 13:31, 18 May 2022
Description
White lumps or powder that is commonly called by its mineral name of Gypsum. Calcium sulfate dihydrate is used in the manufacture of portland cement. Gypsum is also used as a filler and pigment in paints, enamels, glazes and paper.
Synonyms and Related Terms
native calcium sulfate; precipitated calcium sulfate; gypsum; alabaster; selenite; terra alba; satinite; mineral white; satin spar; light spar; Pigment White 25
Risks
- Noncombustible.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water. Slightly soluble in glycerol. Insoluble in most organic solvents. Gypsum fluoresces purple.
| Composition | CaSO4-2H2O |
|---|---|
| CAS | 13397-24-5 |
| Mohs Hardness | 1.5 - 2.0 |
| Melting Point | 100-150 C |
| Density | 2.32-2.36 g/ml |
| Molecular Weight | mol. wt. = 172.2 |
| Refractive Index | 1.520; 1.530; 1.523 |
Comparisons
Characteristics of Common White Pigments
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1753