Difference between revisions of "Chloroform"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A volatile liquid with a characteristic odor. Chloroform was synthesized in 1831 by Liebig and Soubeiran. It was first used as a general anesthetic in 1847 by Sir James Simpson, a physician in Edinburgh. Chloroform is a good [[solvent]] for [[fat|fats]], [[oil|oils]], [[rubber | + | A volatile liquid with a characteristic odor. Chloroform was synthesized in 1831 by Liebig and Soubeiran. It was first used as a general anesthetic in 1847 by Sir James Simpson, a physician in Edinburgh. Chloroform is a good [[solvent]] for [[fat|fats]], [[oil|oils]], [[rubber|rubber]], [[wax|waxes]], and [[natural resin|resins]]. It has been used as a cleaning fluid, refrigerant, degreaser, [[insecticide]], and [[fumigant]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
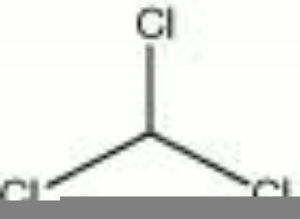
[[[SliderGallery rightalign|chloroform.jpg~Chemical structure]]] | [[[SliderGallery rightalign|chloroform.jpg~Chemical structure]]] | ||
| + | == Risks == | ||
| + | |||
| + | Carcinogen. Toxic by inhalation, ingestion and skin absorption. Nonflammable. | ||
| + | |||
| + | Chloroform can decompose when exposed to heat, moisture and UV light forming highly toxic fumes (phosgene, chlorine gas and hydrogen chloride). Reacts violently with bases, oxidants and some metals (e.g.,aluminium, lithium, magnesium, potassium, sodium), causing fire and explosion hazard. Attacks plastic, rubber and coatings. | ||
| + | |||
| + | ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC610281000&productDescription=CHLOROFORM+ANHYD&vendorId=VN00033901&countryCode=US&language=enttp://www.cdc.gov/niosh/ipcsneng/neng0027.html SDS] | ||
== Other Properties == | == Other Properties == | ||
| Line 36: | Line 43: | ||
| 61.2 | | 61.2 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 49: | Line 48: | ||
[[media:download_file_118.pdf|Properties of Common Solvents]] | [[media:download_file_118.pdf|Properties of Common Solvents]] | ||
| − | + | == Resources and Citations == | |
| − | |||
| − | == | ||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
Revision as of 13:38, 5 August 2020
Description
A volatile liquid with a characteristic odor. Chloroform was synthesized in 1831 by Liebig and Soubeiran. It was first used as a general anesthetic in 1847 by Sir James Simpson, a physician in Edinburgh. Chloroform is a good Solvent for fats, oils, Rubber, waxes, and resins. It has been used as a cleaning fluid, refrigerant, degreaser, Insecticide, and Fumigant.
Synonyms and Related Terms
trichloromethane; trichlormethane (sp); methane trichloride; formyl chloride
Risks
Carcinogen. Toxic by inhalation, ingestion and skin absorption. Nonflammable.
Chloroform can decompose when exposed to heat, moisture and UV light forming highly toxic fumes (phosgene, chlorine gas and hydrogen chloride). Reacts violently with bases, oxidants and some metals (e.g.,aluminium, lithium, magnesium, potassium, sodium), causing fire and explosion hazard. Attacks plastic, rubber and coatings.
ThermoFisher: SDS
Other Properties
Miscible in ethanol, ether, benzene, carbon disulfide. Slightly soluble in water.
| Composition | CHCl3 |
|---|---|
| CAS | 67-66-3 |
| Melting Point | -63.5 |
| Density | 1.485 |
| Molecular Weight | mol. wt. = 119.4 |
| Refractive Index | 1.4422 |
| Boiling Point | 61.2 |
Comparisons
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 186
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993 Comment: bp=61.2C, mp=-63.5C
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2193; bp=61-62C, mp=-63.5C; ref. index= 1.4422
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Encyclopedia Britannica, http://www.britannica.com Comment: "Chloroform." Encyclopædia Britannica. 7 July 2004 .
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index= 1.444