Difference between revisions of "Chromic oxide"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 59: | Line 59: | ||
== Comparisons == | == Comparisons == | ||
| − | [[media: | + | [[media:download_file_209.pdf|Properties of Common Abrasives]] |
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
Revision as of 12:29, 30 May 2020
Description
A dull, olive-green color pigment that occurs naturally as the mineral eskolaite. A process for synthetically producing anhydrous, opaque chrome oxide green was developed in 1809 by Vauquelin. The colorant was listed as an artists pigment in the 1840 Winsor and Newton catalog (Newman 1997). It is opaque, lightfast, and durable with excellent resistance to chemicals and heat. Chromic oxide has limited use in paints because of its dull color. However, it absorbs infrared radiation well and this has led to its use in deck paints and camouflage coatings for military purposed. Chromium oxide is also used as an abrasive, as a glaze color, and for tanning leather.
Synonyms and Related Terms
chromium (III) oxide; chrome oxide; opaque chromium oxide; Pigment Green 17; CI 77288; eskolaite (mineral); óxido de cromo (Esp.); Chromoxidgrün (Deut.); Chromoxid (Deut.); kromivihreä (Fin.); oxyde de chrome (Fr.); vert d'oxyde chrome (Fr.); ossido di cromo (It.); chroomoxidegroen (Ned.); kromoxidgrønn (Nor.); óxido de crómio (Port.); chromium oxide green opaque; chrome sesquioxide; Anadonis green; Schnitzer's green; Reading green; Dingler's green; ultramarine green; leaf green; oil green; green rouge; chromia; ancanthus green --not to be confused with chrome green (lead chromate and Prussian blue) and emerald green (copper acetoarsenite); Schnitzer's green
Other Properties
Particle size 0.1 - 1.0 micrometers. Anisotropic.
Resistant to acids and alkalis. Hexagonal crystal system.
Pleochroism from emerald to olive green.
| Composition | Cr2O3 |
|---|---|
| CAS | 1308-38-9 |
| Melting Point | 2241-2291 |
| Density | 5.10-5.21 |
| Molecular Weight | mol. wt.=151.99 |
| Refractive Index | 2.5 |
| Boiling Point | 4000 |
Hazards and Safety
Toxic by ingestion, inhalation and skin contact.
Suspected carcinogen.
Non-flammable
Mallinckrodt Baker: MSDS
Additional Information
° R. Newman, "Chromium Oxide Greens", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997.° Web Minerals: Eskolaite
Comparisons
Properties of Common Abrasives
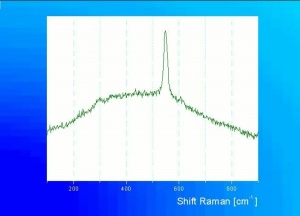
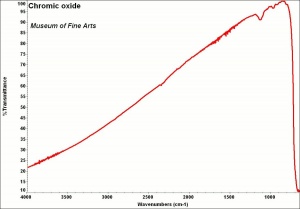
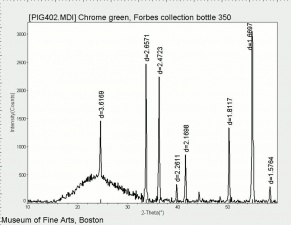
Additional Images
Sources Checked for Data in Record
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: dens=5.10, ref index=2.5
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 193
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Henry Hodges, Artifacts: An Introduction to Early Materials and Technology, Ronald P. Frye, Kingston, Canada, 1988
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2283
- Website address 1 Comment: http://www.coloria.net/varita.htm - foreign language equivalent terms
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Chromium%28III%29_oxide (Accessed Jan. 15, 2006)
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=2.5
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 Comment: numerous synonyms