Difference between revisions of "Copper sulfate, tribasic"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 11: | Line 11: | ||
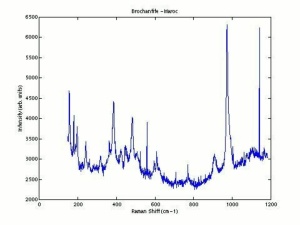
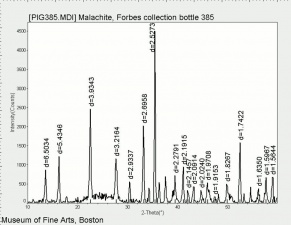
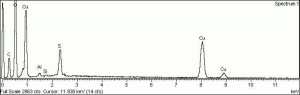
[[[SliderGallery rightalign|Brochantiteitaly1.jpg~Raman|PIG385.jpg~XRD|f385sem.jpg~SEM|f385edsbw.jpg~EDS]]] | [[[SliderGallery rightalign|Brochantiteitaly1.jpg~Raman|PIG385.jpg~XRD|f385sem.jpg~SEM|f385edsbw.jpg~EDS]]] | ||
| + | == Risks == | ||
| − | == | + | Toxic by ingestion. Strongly irritation to skin. |
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in acids. | Soluble in acids. | ||
| Line 25: | Line 28: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry #2723 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry #2723 | ||
| − | |||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 12:07, 27 October 2020
Description
Pale blue or bright green powder that occurs in nature as the minerals Brochantite and langite. Tribasic copper sulfate is one component in Bordeaux mixture that is prepared by mixing Copper sulfate and Copper hydroxide. It is also found as a corrosion product on Copper and Bronze sculptures. Tribasic copper sulfate is used as a Fungicide and Insecticide.
See also Brochantite and Copper sulfate.
Synonyms and Related Terms
copper hydroxide sulfate; cupric subsulfate; Basi-Cop; Cuproxat; Bordeaux mixture; basic copper sulfate; brochantite
Risks
Toxic by ingestion. Strongly irritation to skin.
Physical and Chemical Properties
Soluble in acids.
| Composition | CuSO4-3Cu(OH)2-H2O |
|---|---|
| CAS | 7758-98-7 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry #2723