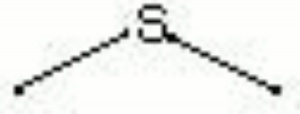Difference between revisions of "Dimethyl sulfide"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 9: | Line 9: | ||
[[[SliderGallery rightalign|dimethyl sulfide.jpg~Chemical structure]]] | [[[SliderGallery rightalign|dimethyl sulfide.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Highly flammable, moderate explosion risk. Flash point = -37C (-35F) | ||
| + | * Evolves sulfur dioxide when heated. | ||
| + | * Contact is very irritating. Inhalation and Ingestion may be harmful. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/23051.htm MSDS] | ||
| + | ==Physical and Chemical Properties== | ||
Soluble in ethanol and ether. Insoluble in water. | Soluble in ethanol and ether. Insoluble in water. | ||
| Line 22: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -83 | + | | -83 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.846 | + | | 0.846 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 37: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 36.2 | + | | 36.2 C |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | == | + | ==Resources and Citations== |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Revision as of 12:19, 21 July 2022
Description
A colorless, foul-smelling liquid that is produced in kraft pulping black liquor. Dimethyl sulfide is used as a Solvent for many mineral salts that are insoluble in water.
Synonyms and Related Terms
methyl sulfide; thiobismethane; 2-thiapropane; dimethyl monosulfide; dimethyl sulphide (Br.); dimethylsulfide; DMS; methyl sulphide (Br.); methyl thioether
Risks
- Highly flammable, moderate explosion risk. Flash point = -37C (-35F)
- Evolves sulfur dioxide when heated.
- Contact is very irritating. Inhalation and Ingestion may be harmful.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in ethanol and ether. Insoluble in water.
| Composition | C2H6S |
|---|---|
| CAS | 75-18-3 |
| Melting Point | -83 C |
| Density | 0.846 g/ml |
| Molecular Weight | mol. wt. = 62.13 |
| Boiling Point | 36.2 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6204