Difference between revisions of "Dimethyl sulfoxide"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 2: | Line 2: | ||
A dense, odorless, colorless liquid used as a solvent and a methylating agent. Dimethyl sulfoxide is obtained as a byproduct from the sulfite pulping paper making procedure. DMSO is used in paint and varnish removers. It is also used in the production of acrylonitrile fibers. | A dense, odorless, colorless liquid used as a solvent and a methylating agent. Dimethyl sulfoxide is obtained as a byproduct from the sulfite pulping paper making procedure. DMSO is used in paint and varnish removers. It is also used in the production of acrylonitrile fibers. | ||
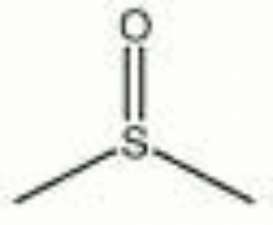
| − | + | [[[SliderGallery rightalign|aaiDMSO.jpg~FTIR|dimethyl sulfoxide.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
DMSO; dimethylsulfoxide; methylsulfoxide; sulfinylbismethane | DMSO; dimethylsulfoxide; methylsulfoxide; sulfinylbismethane | ||
| − | + | == Risks == | |
| − | == | + | * Combustible. Flash point= 89C (192F) |
| + | * Vapors and liquid may be absorbed through the skin causing irritation. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=D1391&productDescription=DIMETHYL+SULFOXIDE+GC+HS+1L&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| + | ==Physical and Chemical Properties== | ||
Soluble in water, ethanol, benzene, acetone, chloroform. | Soluble in water, ethanol, benzene, acetone, chloroform. | ||
| Line 22: | Line 25: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 18.5 | + | | 18.5 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.092 | + | | 1.092 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 37: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 188 | + | | 188 C |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 49: | Line 44: | ||
[[media:download_file_124.pdf|Properties of Common Solvents]] | [[media:download_file_124.pdf|Properties of Common Solvents]] | ||
| − | + | ==Resources and Citations== | |
| − | |||
| − | == | ||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 12:23, 21 July 2022
Description
A dense, odorless, colorless liquid used as a solvent and a methylating agent. Dimethyl sulfoxide is obtained as a byproduct from the sulfite pulping paper making procedure. DMSO is used in paint and varnish removers. It is also used in the production of acrylonitrile fibers.
Synonyms and Related Terms
DMSO; dimethylsulfoxide; methylsulfoxide; sulfinylbismethane
Risks
- Combustible. Flash point= 89C (192F)
- Vapors and liquid may be absorbed through the skin causing irritation.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water, ethanol, benzene, acetone, chloroform.
| Composition | (CH3)2SO |
|---|---|
| CAS | 67-68-5 |
| Melting Point | 18.5 C |
| Density | 1.092 g/ml |
| Molecular Weight | mol. wt. = 78.13 |
| Refractive Index | 1.476 |
| Boiling Point | 188 C |
Comparisons
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.476