Difference between revisions of "Lead tetroxide"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 1: | Line 1: | ||
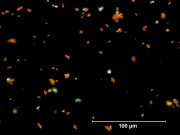
| − | [[File:25_Red_lead_500X.jpg|thumb|Red lead]] | + | [[File:25_Red_lead_500X.jpg|thumb|Red lead at 500x transmitted light]] |
== Description == | == Description == | ||
| − | + | [[File:25_Red_lead_500X_pol.jpg|thumb|Red lead at 500x polarized light]] | |
A heavy, bright red powder that occurs naturally as the mineral [[minium]]. The mineral form, however, was rarely used. Instead lead tetroxide was prepared by slowly heating lead monoxide in a furnace with a constant flow of air. Excess heating will turn the pigment to an orange shade that is known as [[orange mineral]]. Lead tetroxide, also called [[red lead]] oxide, was one of the earliest synthetic pigments. It is no longer used as an artists color because is has poor light stability and poor working properties. Instead, red lead is used to color glass, enamels, and ceramic glazes. It is also used as a flux in porcelain painting, an anticorrosive paint for iron and steel, and as a component in lead batteries. Because of its toxicity, the use of red lead is declining. | A heavy, bright red powder that occurs naturally as the mineral [[minium]]. The mineral form, however, was rarely used. Instead lead tetroxide was prepared by slowly heating lead monoxide in a furnace with a constant flow of air. Excess heating will turn the pigment to an orange shade that is known as [[orange mineral]]. Lead tetroxide, also called [[red lead]] oxide, was one of the earliest synthetic pigments. It is no longer used as an artists color because is has poor light stability and poor working properties. Instead, red lead is used to color glass, enamels, and ceramic glazes. It is also used as a flux in porcelain painting, an anticorrosive paint for iron and steel, and as a component in lead batteries. Because of its toxicity, the use of red lead is declining. | ||
| − | |||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 12: | Line 10: | ||
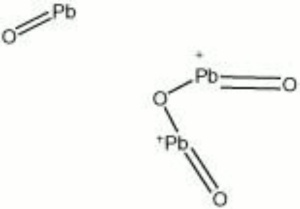
[[[SliderGallery rightalign|lead tetroxide.jpg~Chemical structure]]] | [[[SliderGallery rightalign|lead tetroxide.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Toxic by inhalation or ingestion. | ||
| + | * Skin contact may cause irritation or ulcers. | ||
| + | * Carcinogen, teratogen, suspected mutagen. | ||
| + | * Discolored by hydrogen sulfide. | ||
| + | * ThermoFisher: [https://www.fishersci.com/msds?productName=AC221110050&productD.. SDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Soluble in glacial acetic acid, hot HCl, nitric acid with peroxide present. Insoluble in water and ethanol. | Soluble in glacial acetic acid, hot HCl, nitric acid with peroxide present. Insoluble in water and ethanol. | ||
| Line 25: | Line 31: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 8.32-9.16 | + | | 8.32-9.16 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 40: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | + | * E. West-Fitzhugh, "Red Lead and Minium", ''Artists Pigments'', Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | E. West-Fitzhugh, "Red Lead and Minium", ''Artists Pigments'', Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986. | ||
| − | |||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 8.73 and ref. index = 2.42 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 8.73 and ref. index = 2.42 | ||
| − | * | + | * Pigments Through The Ages - http://webexhibits.org/pigments/indiv/technical/redlead.html - Crystal system: tetragonal Refractive index: 2.42 |
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 56: | Line 52: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5449 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5449 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Lead_tetroxide (Accessed Feb. 2, 2006) |
* Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | ||
Latest revision as of 09:30, 7 October 2022
Description
A heavy, bright red powder that occurs naturally as the mineral Minium. The mineral form, however, was rarely used. Instead lead tetroxide was prepared by slowly heating lead monoxide in a furnace with a constant flow of air. Excess heating will turn the pigment to an orange shade that is known as Orange mineral. Lead tetroxide, also called Red lead oxide, was one of the earliest synthetic pigments. It is no longer used as an artists color because is has poor light stability and poor working properties. Instead, red lead is used to color glass, enamels, and ceramic glazes. It is also used as a flux in porcelain painting, an anticorrosive paint for iron and steel, and as a component in lead batteries. Because of its toxicity, the use of red lead is declining.
Synonyms and Related Terms
red lead; Pigment Red 105; CI 77578; mønje (Dan.); minio (Esp.); tetróxido de chumbo (Port.); minium (Fr.); lead oxide red; lead tetraoxide; orange mineral; Saturn red; Paris red; minio; burnt white lead; red oxide of lead; plumbous plumbate; lead orthoplumbate; mineral red
Risks
- Toxic by inhalation or ingestion.
- Skin contact may cause irritation or ulcers.
- Carcinogen, teratogen, suspected mutagen.
- Discolored by hydrogen sulfide.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in glacial acetic acid, hot HCl, nitric acid with peroxide present. Insoluble in water and ethanol.
| Composition | Pb3O4 |
|---|---|
| CAS | 1314-41-6 |
| Density | 8.32-9.16 g/ml |
| Molecular Weight | mol. wt. = 685.6 |
| Refractive Index | 2.42 |
Resources and Citations
- E. West-Fitzhugh, "Red Lead and Minium", Artists Pigments, Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 8.73 and ref. index = 2.42
- Pigments Through The Ages - http://webexhibits.org/pigments/indiv/technical/redlead.html - Crystal system: tetragonal Refractive index: 2.42
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5449
- Wikipedia: http://en.wikipedia.org/wiki/Lead_tetroxide (Accessed Feb. 2, 2006)
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979