Difference between revisions of "Potassium bicarbonate"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Colorless crystals or white powder. Potassium bicarbonate is used to add [ | + | Colorless crystals or white powder. Potassium bicarbonate is used to add [[carbon%20dioxide|carbon dioxide]] to water, as in soft drinks. It is also used in baking powders, [[fire%20extinguisher|fire extinguishers]], and to adjust the [[pH|pH]] of [[detergent|detergents]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 10:29, 10 May 2016
Description
Colorless crystals or white powder. Potassium bicarbonate is used to add Carbon dioxide to water, as in soft drinks. It is also used in baking powders, fire extinguishers, and to adjust the PH of detergents.
Synonyms and Related Terms
potassium acid carbonate; baking soda
Other Properties
Soluble in water, pH = 8.2 (0.1 M solution). Insoluble in ethanol. Slightly alkaline.
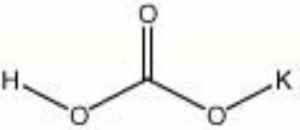
| Composition | KHCO3 |
|---|---|
| CAS | 298-14-6 |
| Melting Point | 100-120 (dec) |
| Density | 2.17 |
| Molecular Weight | mol. wt. = 100.1 |
| Refractive Index | 1.380, 1.482, 1.578 |
Hazards and Safety
Fisher Scientific: MSDS
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7770
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.380, 1.482, 1.578