Difference between revisions of "Sodium acetate"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A colorless, hygroscopic powder. Hydrated sodium acetate is used in photographic emulsions as a [ | + | A colorless, hygroscopic powder. Hydrated sodium acetate is used in photographic emulsions as a [[buffer|buffer]]. It is also used as a [[mordant|mordant]] for dyeing [[textile|textiles]] and as a [[preservative|preservative]] in tanning [[hide|hides]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 12:15, 10 May 2016
Description
A colorless, hygroscopic powder. Hydrated sodium acetate is used in photographic emulsions as a Buffer. It is also used as a Mordant for dyeing textiles and as a Preservative in tanning hides.
Synonyms and Related Terms
sodium acetate trihydrate; Natriumacetat (Deut.); acetato de sódio (Port.)
Other Properties
Soluble in water. Slightly soluble in ethanol. Soluble in ether.
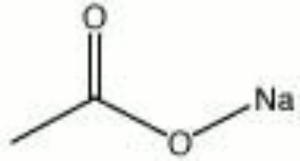
| Composition | NaC2H3O2 |
|---|---|
| CAS | 127-09-3 (anhydrous) |
| Melting Point | 58 |
| Density | 1.45 |
| Molecular Weight | mol. wt. = 82.04 |
| Boiling Point | 324 |
Hazards and Safety
Combustible. Contact may cause irritation.
LINK: International Chemical Safety Card
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8711
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Sodium_acetate (Jan. 6 2006)