Difference between revisions of "Methyl red"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to " $2") |
|||
| Line 4: | Line 4: | ||
[[indicator%20dye|indicator]]. Methyl red is a monoazo dye that was discovered by E. Rupp and R. Loose in 1908. When mixed as a 0.1% alcoholic solution, methyl red has a red color below pH 4.4 and yellow above pH 6.2. It has been used for titrating | [[indicator%20dye|indicator]]. Methyl red is a monoazo dye that was discovered by E. Rupp and R. Loose in 1908. When mixed as a 0.1% alcoholic solution, methyl red has a red color below pH 4.4 and yellow above pH 6.2. It has been used for titrating | ||
[[ammonium%20hydroxide|ammonia]] and weak organic bases, but it is not suitable for titrating acids. Currently, methyl red is rarely used because it is easily reduced to a colorless form. | [[ammonium%20hydroxide|ammonia]] and weak organic bases, but it is not suitable for titrating acids. Currently, methyl red is rarely used because it is easily reduced to a colorless form. | ||
| − | + | [[[SliderGallery rightalign|methyl red.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Acid Red 2; CI 13020; 2-[[(4-dimethylamino)phenyl]-azo]benzoic acid; 4'-dimethylaminoazobenzene-2-carboxylic acid; rojo de metilo (Esp.); rouge méthyle (Fr.); vermelho de metilo (Port.) | Acid Red 2; CI 13020; 2-[[(4-dimethylamino)phenyl]-azo]benzoic acid; 4'-dimethylaminoazobenzene-2-carboxylic acid; rojo de metilo (Esp.); rouge méthyle (Fr.); vermelho de metilo (Port.) | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Toxic by ingestion and inhalation. | ||
| + | * Contact may cause irritation | ||
| + | * Potential carcinogen. | ||
| + | * Flammable. Flash point = 12 C | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/96266.htm MSDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in ethanol and acetic acid. Insoluble in water. | Soluble in ethanol and acetic acid. Insoluble in water. | ||
| Line 24: | Line 30: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 181-182 | + | | 181-182 C |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 30: | Line 36: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 184 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 184 | ||
Latest revision as of 13:28, 1 October 2022
Description
A reddish-violet powder that was formerly used as an acid-base
indicator. Methyl red is a monoazo dye that was discovered by E. Rupp and R. Loose in 1908. When mixed as a 0.1% alcoholic solution, methyl red has a red color below pH 4.4 and yellow above pH 6.2. It has been used for titrating
ammonia and weak organic bases, but it is not suitable for titrating acids. Currently, methyl red is rarely used because it is easily reduced to a colorless form.
Synonyms and Related Terms
Acid Red 2; CI 13020; 2-[[(4-dimethylamino)phenyl]-azo]benzoic acid; 4'-dimethylaminoazobenzene-2-carboxylic acid; rojo de metilo (Esp.); rouge méthyle (Fr.); vermelho de metilo (Port.)
Risks
- Toxic by ingestion and inhalation.
- Contact may cause irritation
- Potential carcinogen.
- Flammable. Flash point = 12 C
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in ethanol and acetic acid. Insoluble in water.
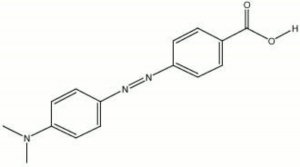
| Composition | (CH3)2NC6H4NNC6H4COOH |
|---|---|
| CAS | 493-52-7 |
| Melting Point | 181-182 C |
| Molecular Weight | mol. wt. = 269.29 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 184
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6199
- S.R.Trotman, E.R. Trotman, Textile Analysis, J.B. Lippincott Company, Philadelphia, 1932
- Colour Index International online at www.colour-index.org