Difference between revisions of "Methyl violet"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to " $2") |
|||
| Line 35: | Line 35: | ||
== Hazards and Safety == | == Hazards and Safety == | ||
| − | + | Fisher Scientific: [https://www.fishersci.com/store/msds?partNumber=S25436A&productDescription=METHYL+VIOLET+2B+25G&vendorId=VN00115888&countryCode=US&language=en MSDS] | |
== Sources Checked for Data in Record == | == Sources Checked for Data in Record == | ||
Revision as of 07:52, 25 September 2019
Description
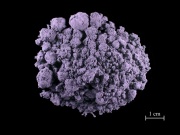
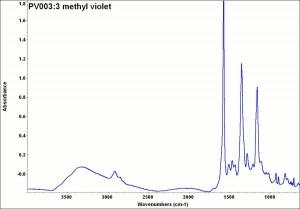
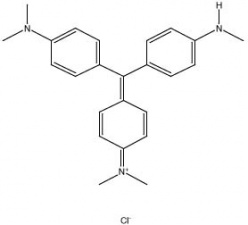
A blue-green powder that forms a deep violet aqueous solution. Methyl violet is a triphenylmethane dye that was synthesized in 1861 by Lauth. It has a very strong tinting strength and is used as a dye for Wood, Silk, and Paper. Methyl violet is also used in inks, as a biological stain, and as an acid-base indicator.
Synonyms and Related Terms
Basic Violet 1; CI 42535; Methyl Violet 2B; Solvent Violet 8 (base); Pigment Violet 3 (phosphotungstomolybdic acid salt); Methylviolett (Deut.); méthyl violet (Fr.); violeta de metilo (Esp., Port.); violetto metile (It.); methylviolet (Ned.)
Other Properties
Soluble in water, chloroform. Lightly soluble in ethanol, glycerol. Insoluble in ether.
| Composition | C24H28ClN3 |
|---|---|
| CAS | 8004-87-3 |
| Melting Point | 137 |
| Molecular Weight | mol. wt. = 393.96 |
Hazards and Safety
Fisher Scientific: MSDS
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4401
- Website address 1 Comment: www.straw.com/sig/dyehist
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Colour Index International online at www.colour-index.org