Difference between revisions of "Silver nitrate"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Colorless, corrosive crystals that darken on exposure to light. Silver nitrate is used primarily in manufacturing photographic emulsions and silver mirrors. It is also used in | + | Colorless, corrosive crystals that darken on exposure to light. Silver nitrate is used primarily in manufacturing photographic emulsions and silver mirrors. It is also used in silver plating, dyeing hair, etching [[ivory|ivory]], and as a colorant for [[porcelain|porcelain]] and [[glass|glass]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 8: | Line 8: | ||
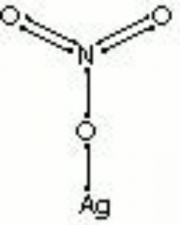
[[[SliderGallery rightalign|silver nitrate.jpg~Chemical structure]]] | [[[SliderGallery rightalign|silver nitrate.jpg~Chemical structure]]] | ||
| + | == Risks == | ||
| − | == | + | Strong oxidizing agent. Toxic by ingestion and inhalation. Corrosive to skin and mucous membranes. |
| + | |||
| + | Fisher Scientific: [https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-s/S25526.pdf SDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in water, glycerol, hot ethanol. Slightly soluble in ether. | Soluble in water, glycerol, hot ethanol. Slightly soluble in ether. | ||
| Line 39: | Line 43: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 725 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 725 | ||
| Line 57: | Line 55: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8661 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8661 | ||
| − | * Website | + | * Website: www.jetcity.com/~mrjones/chemdesc.htm - photographic chemicals |
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.729, 1.744, 1.788 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.729, 1.744, 1.788 | ||
Revision as of 11:02, 8 December 2020
Description
Colorless, corrosive crystals that darken on exposure to light. Silver nitrate is used primarily in manufacturing photographic emulsions and silver mirrors. It is also used in silver plating, dyeing hair, etching Ivory, and as a colorant for Porcelain and Glass.
Synonyms and Related Terms
lapis infernis; lunar caustic; sølvnitrat (Dan.); Silbernitrat (Deut.); nitrato de plata (Esp.); nitrato d'argento (It.); zilvernitraat (Ned.); Azotan(V) srebra(I)(Pol.); sølvnitrat (Nor.)
Risks
Strong oxidizing agent. Toxic by ingestion and inhalation. Corrosive to skin and mucous membranes.
Fisher Scientific: SDS
Physical and Chemical Properties
Soluble in water, glycerol, hot ethanol. Slightly soluble in ether.
Tabular, rhombic shaped crystals.
| Composition | AgNO3 |
|---|---|
| CAS | 7761-88-8 |
| Melting Point | 212 |
| Density | 4.328 |
| Molecular Weight | mol. wt. = 169.9 |
| Refractive Index | 1.729, 1.744, 1.788 |
| Boiling Point | 444 (dec) |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 725
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8661
- Website: www.jetcity.com/~mrjones/chemdesc.htm - photographic chemicals
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.729, 1.744, 1.788