Difference between revisions of "Tartaric acid"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 9: | Line 9: | ||
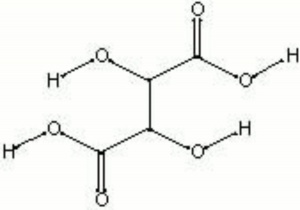
[[[SliderGallery rightalign|tartaric acid.jpg~Chemical structure]]] | [[[SliderGallery rightalign|tartaric acid.jpg~Chemical structure]]] | ||
| − | == | + | == Physical and Chemical Properties == |
| − | Soluble in water, ethanol, ether, glycerol. Insoluble in chloroform. | + | * Soluble in water, ethanol, ether, glycerol. Insoluble in chloroform. |
| − | + | * Smells of burnt sugar when heated. | |
| − | Smells of burnt sugar when heated. | + | * pH = 2.2 (0.1 N solution) |
| − | |||
| − | pH = 2.2 (0.1 N solution) | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 26: | Line 24: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 170 | + | | 170 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.76 | + | | 1.76 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 35: | Line 33: | ||
|} | |} | ||
| − | == | + | == Risks == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * Combustible. Flash point = 210 C | |
| + | * Corrosive. | ||
| + | * Contact cause irritation and burns. | ||
| + | * Fisher Scientific: [https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-t/S25601A.pdf SDS] | ||
| − | == | + | ==Resources and Citations== |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 68 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 68 | ||
| Line 51: | Line 48: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9235 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9235 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Tartaric_acid (Accessed Sept. 28, 2005) |
* S.R.Trotman, E.R. Trotman, ''Textile Analysis'', J.B. Lippincott Company, Philadelphia, 1932 | * S.R.Trotman, E.R. Trotman, ''Textile Analysis'', J.B. Lippincott Company, Philadelphia, 1932 | ||
| Line 63: | Line 60: | ||
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: pH = 2.2 (0.1 N solution) | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: pH = 2.2 (0.1 N solution) | ||
| − | * | + | * Photographic chemicals: www.jetcity.com/~mrjones/chemdesc.htm |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 07:54, 8 June 2022
Description
Colorless, transparent crystals that occur naturally in wine lees. Tartaric acid is used in baking powders (potassium hydrogen tartrate), leather tanning and effervescent beverages. Tartaric acid acts as a buffering agent and Sequestrant. It is used by dyers to print a blue ferric tartrate color and to remove some mordants from solution. Tartaric acid (and tartrates) reacts with ammonio-silver nitrate to produce metallic Silver; this reaction is used in photographic developing solutions and for silvering mirrors.
Synonyms and Related Terms
tartar; dihydroxysuccinic acid; L-tartaric acid; racemic acid; uvic acid; Weinsäure (Deut.); Ácido tartárico (Esp., Port.); Wijnsteenzuur (Ned.)
Physical and Chemical Properties
- Soluble in water, ethanol, ether, glycerol. Insoluble in chloroform.
- Smells of burnt sugar when heated.
- pH = 2.2 (0.1 N solution)
| Composition | HOOC(CH2O)2COOH |
|---|---|
| CAS | 133-37-9 |
| Melting Point | 170 C |
| Density | 1.76 g/ml |
| Molecular Weight | mol. wt. = 150.1 |
Risks
- Combustible. Flash point = 210 C
- Corrosive.
- Contact cause irritation and burns.
- Fisher Scientific: SDS
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 68
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9235
- Wikipedia: http://en.wikipedia.org/wiki/Tartaric_acid (Accessed Sept. 28, 2005)
- S.R.Trotman, E.R. Trotman, Textile Analysis, J.B. Lippincott Company, Philadelphia, 1932
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: pH = 2.2 (0.1 N solution)
- Photographic chemicals: www.jetcity.com/~mrjones/chemdesc.htm