Difference between revisions of "Quinacridone dye"
| Line 18: | Line 18: | ||
{| class="wikitable" | {| class="wikitable" | ||
| + | ! Pigment number !! Manufacture !! Pigment name !! Manufacture CI number !! Comments | ||
| + | |- | ||
|- | |- | ||
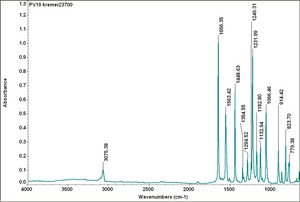
| PV019 || Kremer|| unspecified || 23700 || | | PV019 || Kremer|| unspecified || 23700 || | ||
|- | |- | ||
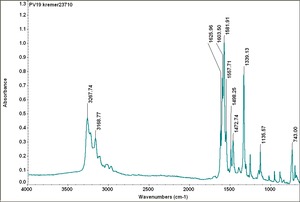
| PV019 || Kremer|| unspecified || 23710 || same as PV019 Sun quinacradone violet 228-1119 | | PV019 || Kremer|| unspecified || 23710 || same as PV019 Sun quinacradone violet 228-1119 | ||
| − | |||
| − | |||
|- | |- | ||
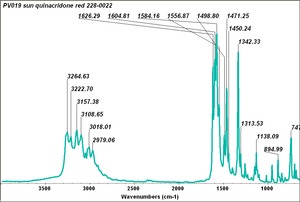
| PV019|| Sun || quinacradone red|| 228-0022 || | | PV019|| Sun || quinacradone red|| 228-0022 || | ||
Revision as of 12:22, 28 January 2020
Description
A series of synthetic red and violet dyes composed of linear quinacridones that are made from terephthalic acid. Quinacridone dyes can exist in four crystalline allotropes, two of which are sold as red and violet pigments. Although synthesized in 1896, quinacridone colors were first recognized as useful pigments by W. Struve at DuPont in 1955 and marketed in 1958 under the name Monastral. These lightfast colorants are used in paints, printing inks, and plastics.
Synonyms and Related Terms
colorante de quinacridona (Esp.)
Examples include:
Red - Monastral red [DuPont; Acra red [Liquitex]; Acra crimson [Binney and Smith]; Bocour red [Bocour Artist Colors];Thalo Red Rose
Violet - Monastral violet, Pigment Violet 19; CI 46500;
Comparisons
| Pigment number | Manufacture | Pigment name | Manufacture CI number | Comments |
|---|---|---|---|---|
| PV019 | Kremer | unspecified | 23700 | |
| PV019 | Kremer | unspecified | 23710 | same as PV019 Sun quinacradone violet 228-1119 |
| PV019 | Sun | quinacradone red | 228-0022 | |
| PV019 | Sun | quinacradone violet | 228-1119 | same as PV019 Kremer 23710 |
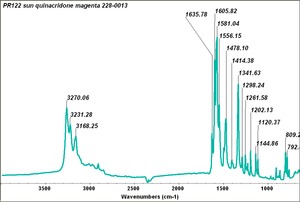
| PR122 | Sun | quinacradone magenta | 228-0013 | |
| PV122 | Kremer | unspecified | 23152 | |
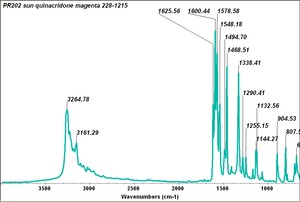
| PR202 | Sun | quinacradone magenta | 228-1215 |
Other Properties
Resistant to alkalis and heat.
| Composition | violet- C20H12N2O2 |
|---|---|
| Density | 1.5 |
| Refractive Index | 2.02 - 2.04 |
Sources Checked for Data in Record
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 611
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Monona Rossol, The Artist's Complete Health and Safety Guide, Allworth Press, New York, 1994
- Encyclopedia Britannica, http://www.britannica.com Comment: "chemical compound." Encyclopædia Britannica. 2005. Encyclopædia Britannica Premium Service 7 Apr. 2005 .
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Website address 1 Comment: www.handprint.com