Difference between revisions of "Naphthol pigment"
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A general class of azo-type organic colorants. Naphthol pigments contain a backbone of 2-hydroxy-3-naphthanilide (Napthol AS) and include many shades of red and some oranges. They were first patented in 1911 and sold as Grela Reds but did not become popularly used until the 1930s. About 6% of the red colorants used in 1985 were naphthol pigments. They provide good tinting strength and good lightfastness, but can bleed when exposed to organic solvents. Naphthol reds are used to color plastics, automotive finishes, architectural paints, pencils, crayon, printing inks and inexpensive artists oil paints and watercolors | + | A general class of azo-type organic colorants. Naphthol pigments contain a backbone of 2-hydroxy-3-naphthanilide (Napthol AS) and include many shades of red and some oranges. They were first patented in 1911 and sold as Grela Reds but did not become popularly used until the 1930s. About 6% of the red colorants used in 1985 were naphthol pigments. They provide good tinting strength and good lightfastness, but can bleed when exposed to organic solvents. Naphthol reds are used to color plastics, automotive finishes, architectural paints, pencils, crayon, printing inks and inexpensive artists oil paints and watercolors. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Latest revision as of 08:22, 30 October 2020
Description
A general class of azo-type organic colorants. Naphthol pigments contain a backbone of 2-hydroxy-3-naphthanilide (Napthol AS) and include many shades of red and some oranges. They were first patented in 1911 and sold as Grela Reds but did not become popularly used until the 1930s. About 6% of the red colorants used in 1985 were naphthol pigments. They provide good tinting strength and good lightfastness, but can bleed when exposed to organic solvents. Naphthol reds are used to color plastics, automotive finishes, architectural paints, pencils, crayon, printing inks and inexpensive artists oil paints and watercolors.
Synonyms and Related Terms
naphthol dye; azoic dye; Naphthol Red; Permanent Carmine; Grela Red; napthol dye (sp); hydroxynaphthoic anilide; Naphtol Anilid Saure (Deut.); Napthol AS (Deut.); pigment à base de naphtol (Fr.);
Comparisons
| Pigment number | Manufacture | Pigment name | Manufacture CI number | Comments |
|---|---|---|---|---|
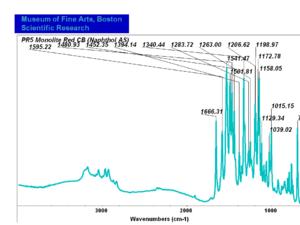
| PR005 | unknown | monolite red CB | unknown | |
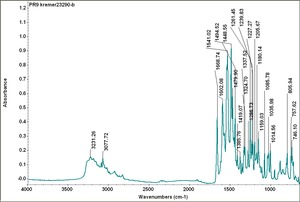
| PR009 | Kremer | unidentified | 23290 | |
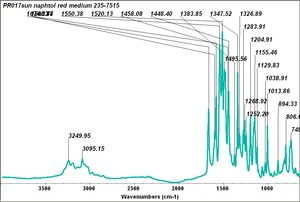
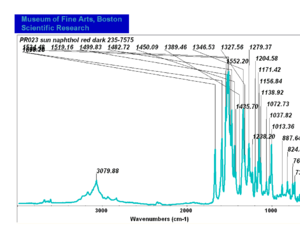
| PR017 | Sun | napthol red medium | 235-7515 | |
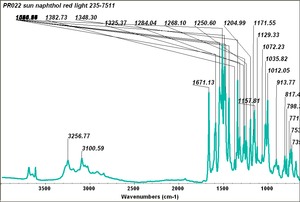
| PR022 | Sun | napthol red light | 235-7511 | |
| PR022 | Magruder | napthol red light shade | ra1307-dc | |
| PR023 | Sun | napthol red dark | 235-7575 | |
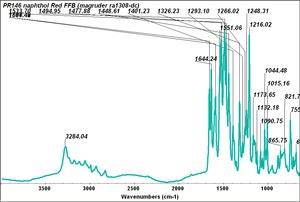
| PR146 | Magruder | napthol red FFB | ra1308-dc | |
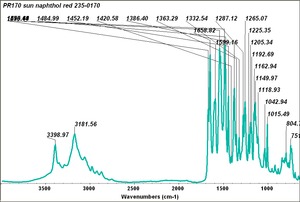
| PR170 | Sun | napthol red | 235-0170 | |
| PR170 | Kremer | napthol red | 23290-a | |
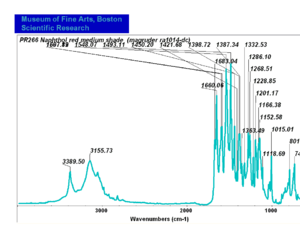
| PR266 | Magruder | napthol red medium shade | ra1014-dc | |
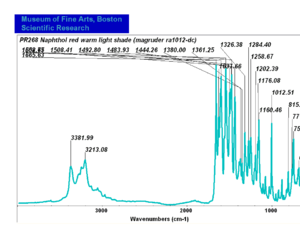
| PR268 | Magruder | napthol red warm light shade | ra1012-dc | |
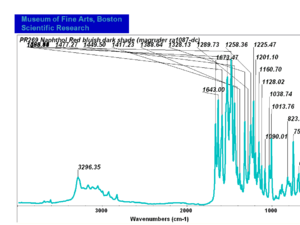
| PR269 | Magruder | napthol red bluish dark shade | ra1087-dc |
Physical and Chemical Properties
Resistant to acid, alkali and soap.
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993 Comment: Napthol AS = azoic coupling component
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- Rosalie Rosso King, Textile Identification, Conservation, and Preservation, Noyes Publications, Park Ridge, NJ, 1985
- Fairchild's Dictionary of Textiles, Phyllis G.Tortora, Robert S. Merkel (eds.), Fairchild Publications, New York City, 7th edition, 1996
- Encyclopedia Britannica, http://www.britannica.com Comment: "Chemical Compound." (Accessed 13 May 2004). gives date of first use as 1912
- B. Berrie, S.Q. Lomax, 'Azo Pigments: Their History, Synthesis, Properties and Use in Artists' Materials', Studies in the History of Art , National Gallery of Art, Washington DC, No. 57, 1997 Comment: patented 1911