Difference between revisions of "Lead oxychloride"
m (added image) |
(revised microscopic characteristics) |
||
| Line 17: | Line 17: | ||
==Microscopic Characteristics== | ==Microscopic Characteristics== | ||
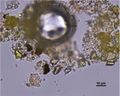
| − | In PPL, particles have a lemon yellow color with moderate to high relief. | + | In PPL, particles have a pale to medium lemon-yellow color with moderate to high relief. The particle size distribution is typically very coarse, with larger particles measuring as large as 15 microns. Smaller particles can appear shard-like, sometimes with conchoidal fracture, while larger particles can appear somewhat platy with a distinctive shallow stair-step pattern along some edges. RI > 1.662. |
| + | In XPL, this pigment is reported (Eastaugh 693) as being highly birefringent with third and fourth order colors observed, but many particles have low first order birefringence, or appear dark with a yellow birefringence along the grain boundaries. Distinctively, some particles may show blue/violet interference colors. | ||
== Risks == | == Risks == | ||
Revision as of 09:56, 25 March 2022
Description
A strong yellow pigment that was also called Turner's yellow. Lead oxychloride was discovered in 1770 by Scheele but not patented until 1781 by J. Turner in England. Turner's yellow was made by mixing powdered lead chloride with lead carbonate then fusing. This yellow pigment was used for a short time in oil paints, but its sensitivity to light and sulfur fumes limited its use and it was replaced by chrome yellow pigments.
Synonyms and Related Terms
hydrated lead chloride; patent yellow (PbCl2.5-7PbO); Montpelier yellow; Kassel yellow; mineral yellow; Vernona yellow; Cassel yellow (PbCl2-7PbO); Turner's yellow
| Composition | PbCl2.5-7PbO (patent yellow) |
|---|
Microscopic Characteristics
In PPL, particles have a pale to medium lemon-yellow color with moderate to high relief. The particle size distribution is typically very coarse, with larger particles measuring as large as 15 microns. Smaller particles can appear shard-like, sometimes with conchoidal fracture, while larger particles can appear somewhat platy with a distinctive shallow stair-step pattern along some edges. RI > 1.662. In XPL, this pigment is reported (Eastaugh 693) as being highly birefringent with third and fourth order colors observed, but many particles have low first order birefringence, or appear dark with a yellow birefringence along the grain boundaries. Distinctively, some particles may show blue/violet interference colors.
Risks
Turns black when exposed to water, UV light and sulfur fumes.
Additional Images
Resources and Citations
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- R. Newman, E. Farrell, 'House Paint Pigments', Paint in America , R. Moss ed., Preservation Press, New York City, 1994
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: first prepared in 1775
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments" (gives date of 1781 for patent)
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004