Difference between revisions of "Potassium persulfate"
Jump to navigation
Jump to search
| Line 2: | Line 2: | ||
White, odorless crystals. Potassium persulfate is used to [[bleaching%20agent|bleach]] and de-[[size|size]] textiles. It is used in photographic solution as a hypo eliminator to remove [[sodium%20thiosulfate|sodium thiosulfate]] from the plates and paper. | White, odorless crystals. Potassium persulfate is used to [[bleaching%20agent|bleach]] and de-[[size|size]] textiles. It is used in photographic solution as a hypo eliminator to remove [[sodium%20thiosulfate|sodium thiosulfate]] from the plates and paper. | ||
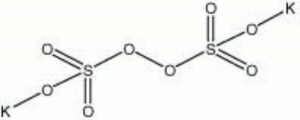
| − | + | [[[SliderGallery rightalign|potassium persulfate.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
potassium persulphate (Br.); potassium peroxydisulfate; persulfate of potash; Anthion | potassium persulphate (Br.); potassium peroxydisulfate; persulfate of potash; Anthion | ||
| − | |||
| − | |||
== Risks == | == Risks == | ||
Latest revision as of 11:31, 25 August 2022
Description
White, odorless crystals. Potassium persulfate is used to bleach and de-Size textiles. It is used in photographic solution as a hypo eliminator to remove Sodium thiosulfate from the plates and paper.
Synonyms and Related Terms
potassium persulphate (Br.); potassium peroxydisulfate; persulfate of potash; Anthion
Risks
- Strong oxidizing agent.
- Skin contact causes irritation.
- Toxic by ingestion.
- Reacts violently with organics and reducing salts.
- Releases sulfur dioxide gas with decomposition.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water. Insoluble in ethanol.
| Composition | K2S2O8 |
|---|---|
| CAS | 7727-21-1 |
| Density | 2.477 g/ml |
| Molecular Weight | mol. wt. = 270.32 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 163
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7825
- Photographic chemicals: www.jetcity.com/~mrjones/chemdesc.htm