Difference between revisions of "Aluminum sulfate"
| Line 24: | Line 24: | ||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 33 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 33 | ||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | |||
* Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | ||
| − | |||
* E.J.LaBarre, ''Dictionary and Encyclopedia of Paper and Paper-making'', Swets & Zeitlinger, Amsterdam, 1969 | * E.J.LaBarre, ''Dictionary and Encyclopedia of Paper and Paper-making'', Swets & Zeitlinger, Amsterdam, 1969 | ||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 381 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 381 | ||
| − | + | * Wikipedia: [https://en.wikipedia.org/wiki/Aluminium_sulfate Aluminum_sulfate] (Accessed Mar. 15, 2006 and March 2025) | |
| − | * Wikipedia: | ||
| − | |||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| − | |||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | |||
* Rosalie Rosso King, ''Textile Identification, Conservation, and Preservation'', Noyes Publications, Park Ridge, NJ, 1985 | * Rosalie Rosso King, ''Textile Identification, Conservation, and Preservation'', Noyes Publications, Park Ridge, NJ, 1985 | ||
| − | |||
* Boise Cascade Paper Group, ''The Paper Handbook'', Boise Cascade, Portland OR, 1989 | * Boise Cascade Paper Group, ''The Paper Handbook'', Boise Cascade, Portland OR, 1989 | ||
| − | |||
* Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | ||
| − | |||
* Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 | * Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 | ||
Latest revision as of 10:29, 16 March 2025
Description
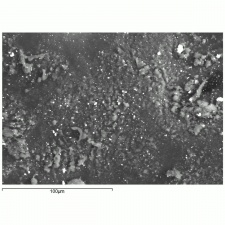
A white crystalline compound that has historically been called papermakers' alum. Aluminum sulfate occurs in nature as the mineral alunogenite. It is prepared synthetically by treating Bauxite with Sulfuric acid. Starting in the 1820s, aluminum sulfate began to be used for sizing paper. It reacts with Rosin causing it to flocculate to the cellulosic fibers in the pulp solution. However, residual alum in the paper produces an acidic environment that will accelerated degradation of paper. Aluminum sulfate has also been used to taw leather, as a dye mordant, as a substrate for lake pigments, for waterproofing concrete, and for fireproofing textiles. It is also used as a flocculant in water purification systems.
Synonyms and Related Terms
aluminium sulfate (IUPAC): aluminium sulphate (Br.); Aluminiumsulfat (Deut.); sulfate d'aluminium (Fr.); aluminum trisulfate; alum; pearl alum; pickle alum; cake alum; filter alum; papermakers alum; patent alum
Risks
- Noncombustible.
- Fisher Scientific: SDS
Physical and Chemical Properties
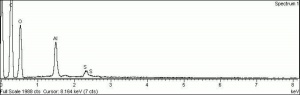
- Composition = Al2(SO4)3. 16H20 (mol. wt. = 630.40)
- CAS = 10043-01-3 (anh.) 16828-11-8 (hyd.)
- Melting Point = 770 C (dec.)
- Density = 2.71 g/ml (hydrate)
- Soluble in water. Insoluble in ethanol.
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 33
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- E.J.LaBarre, Dictionary and Encyclopedia of Paper and Paper-making, Swets & Zeitlinger, Amsterdam, 1969
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 381
- Wikipedia: Aluminum_sulfate (Accessed Mar. 15, 2006 and March 2025)
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Rosalie Rosso King, Textile Identification, Conservation, and Preservation, Noyes Publications, Park Ridge, NJ, 1985
- Boise Cascade Paper Group, The Paper Handbook, Boise Cascade, Portland OR, 1989
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986