Difference between revisions of "Aldrin"
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
| − | |||
== Description == | == Description == | ||
| Line 8: | Line 7: | ||
HHDN; Octalene [Hyman & Co.]; Compound 118; 1,2,3,4,10,10-hexachloro- 1,4,4a,5,8,8a-hexahydro-1,4:5,8-dimethanonaphthalene | HHDN; Octalene [Hyman & Co.]; Compound 118; 1,2,3,4,10,10-hexachloro- 1,4,4a,5,8,8a-hexahydro-1,4:5,8-dimethanonaphthalene | ||
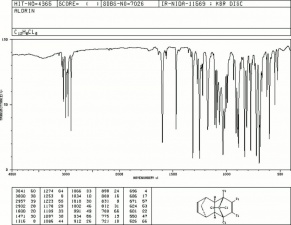
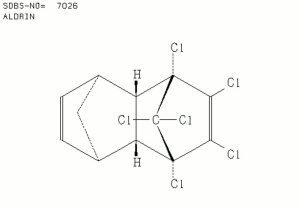
| − | [[[SliderGallery rightalign|aldrinstructure.jpg~Chemical structure]]] | + | [[[SliderGallery rightalign|aldrinir.jpg~FTIR|aldrinstructure.jpg~Chemical structure]]] |
== Other Properties == | == Other Properties == | ||
| Line 42: | Line 41: | ||
== Authority == | == Authority == | ||
| − | * | + | * Nancy Odegaard, Alyce Sadongei, and associates, ''Old Poisons, New Problems'', Altimira, Walnut Creek, CA, 2005 |
* ''Encyclopedia Britannica'', http://www.britannica.com Comment: history of agriculture [Accessed March 26, 2002] for date of introduction | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: history of agriculture [Accessed March 26, 2002] for date of introduction | ||
| − | * | + | * G.Caneva, M.P.Nugari, O.Salvadori, ''Biology in the Conservation of Works of Art'', ICCROM, Rome, 1991 |
| − | * | + | * Stephen R. Edwards, Bruce M. Bell, Mary Elizabeth King, ''Pest Control in Museums: a Status Report 1980'', Association of Sytematics Collections, Washington DC, 1980 |
| − | * | + | * Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 Comment: Interior use |
* Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Aldrin (Accessed Mar. 15, 2006) | * Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Aldrin (Accessed Mar. 15, 2006) | ||
Revision as of 06:21, 24 July 2013
Description
A toxic, chlorinated hydrocarbon that occurs as white to brown crystals. Aldrin was first introduced as an insecticide in 1948. The former insecticide was primarily used for cockroaches, subterranean termites, and mothproofing. However, it irreversibly reacts with keratin and other proteins. Because of its toxicity, it is no longer manufactured or used in the US since 1987.
Synonyms and Related Terms
HHDN; Octalene [Hyman & Co.]; Compound 118; 1,2,3,4,10,10-hexachloro- 1,4,4a,5,8,8a-hexahydro-1,4:5,8-dimethanonaphthalene
Other Properties
Very soluble in most organic solvents. Insoluble in water.
| Composition | C12H8Cl6 |
|---|---|
| CAS | 309-00-2 |
| Melting Point | 104 |
| Molecular Weight | mol. wt. = 364.9 |
| Boiling Point | 145 |
Hazards and Safety
Extremely hazardous. Toxic by inhalation, skin absorption, or ingestion.
LD50=36-60 mg/kg. Suspected teratogen and carcinogen.
International Chemical Safety Card
Authority
- Nancy Odegaard, Alyce Sadongei, and associates, Old Poisons, New Problems, Altimira, Walnut Creek, CA, 2005
- Encyclopedia Britannica, http://www.britannica.com Comment: history of agriculture [Accessed March 26, 2002] for date of introduction
- G.Caneva, M.P.Nugari, O.Salvadori, Biology in the Conservation of Works of Art, ICCROM, Rome, 1991
- Stephen R. Edwards, Bruce M. Bell, Mary Elizabeth King, Pest Control in Museums: a Status Report 1980, Association of Sytematics Collections, Washington DC, 1980
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002 Comment: Interior use
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Aldrin (Accessed Mar. 15, 2006)