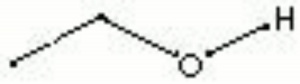Difference between revisions of "Ethyl alcohol"
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A clear, colorless, [http://cameo.mfa.org/materials/fullrecord.asp?name=hygroscopic hygroscopic] liquid with a pleasant odor. Ethyl alcohol, or ethanol, is primarily used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=solvent solvent] and as an intoxicating beverage. Ethanol forms a binary [http://cameo.mfa.org/materials/fullrecord.asp?name=azeotrope azeotrope] with water that boils at 78.15C and contains 95.57% ethanol and 4.43% water. The addition of [http://cameo.mfa.org/materials/fullrecord.asp?name=benzene benzene] allows the mixture to be redistilled without the water. Ethyl alcohol is sold in many grades marked as 95%, absolute (100% or anhydrous), denatured, industrial, or listed as proofs (one-half the proof is the percentage of alcohol). In art and conservation, ethanol has been used as a solvent for [http://cameo.mfa.org/materials/fullrecord.asp?name=shellac shellac] and [http://cameo.mfa.org/materials/fullrecord.asp?name=mastic | + | A clear, colorless, [http://cameo.mfa.org/materials/fullrecord.asp?name=hygroscopic hygroscopic] liquid with a pleasant odor. Ethyl alcohol, or ethanol, is primarily used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=solvent solvent] and as an intoxicating beverage. Ethanol forms a binary [http://cameo.mfa.org/materials/fullrecord.asp?name=azeotrope azeotrope] with water that boils at 78.15C and contains 95.57% ethanol and 4.43% water. The addition of [http://cameo.mfa.org/materials/fullrecord.asp?name=benzene benzene] allows the mixture to be redistilled without the water. Ethyl alcohol is sold in many grades marked as 95%, absolute (100% or anhydrous), denatured, industrial, or listed as proofs (one-half the proof is the percentage of alcohol). In art and conservation, ethanol has been used as a solvent for [http://cameo.mfa.org/materials/fullrecord.asp?name=shellac shellac] and [http://cameo.mfa.org/materials/fullrecord.asp?name=mastic%20resin mastic], as a diluent for [http://cameo.mfa.org/materials/fullrecord.asp?name=fixative fixatives], and as a [http://cameo.mfa.org/materials/fullrecord.asp?name=wetting%20agent wetting agent]. When used as a solvent for resins, the ethanol must be dry because any moisture will produce a white haze in the varnish film. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 53: | Line 53: | ||
== Authority == | == Authority == | ||
| − | * | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 |
| − | * | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3806 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3806 | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.359 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.359 | ||
Revision as of 06:23, 24 July 2013
Description
A clear, colorless, hygroscopic liquid with a pleasant odor. Ethyl alcohol, or ethanol, is primarily used as a solvent and as an intoxicating beverage. Ethanol forms a binary azeotrope with water that boils at 78.15C and contains 95.57% ethanol and 4.43% water. The addition of benzene allows the mixture to be redistilled without the water. Ethyl alcohol is sold in many grades marked as 95%, absolute (100% or anhydrous), denatured, industrial, or listed as proofs (one-half the proof is the percentage of alcohol). In art and conservation, ethanol has been used as a solvent for shellac and mastic, as a diluent for fixatives, and as a wetting agent. When used as a solvent for resins, the ethanol must be dry because any moisture will produce a white haze in the varnish film.
Synonyms and Related Terms
ethanol (IUPAC); alcohol; grain alcohol; absolute alcohol, EtOH, anhydrous alcohol; dehydrated alcohol; ethyl hydrate; ethyl hydroxide; Cologne spirits; colonial spirits; rectified spirits; spirits of wine; fermentation alcohol
Other Properties
Miscible with water, methanol, ether, chloroform, acetone.
| Composition | C2H5OH |
|---|---|
| CAS | 64-17-5 |
| Melting Point | -114.1 |
| Density | 0.789 |
| Molecular Weight | mol. wt.= 46.08 |
| Refractive Index | 1.359 |
| Boiling Point | 78.5 |
Hazards and Safety
Highly flammable. Flash point = 14 C (60F).
Inhalation, and skin contact can cause irritation. Ingestion of small amounts affects the central nervous system. Ingestion of large amounts is deadly.
International Chemical Safety Card
Comparisons
Authority
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3806
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.359