Difference between revisions of "Acridine yellow"
(username removed) |
(username removed) |
||
| Line 45: | Line 45: | ||
== Authority == | == Authority == | ||
| − | * | + | * Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, ''Notes for Workshop on New Methods in the Cleaning of Paintings'', J.Paul Getty Trust, Los Angeles, 1990 |
| − | * | + | * Website address 1 Comment: Fisher Scientific at: https://www1.fishersci.com/catalogs/acrosgroup.jsp?catalogParamId=8013896&catalogParamType=AG mp = >400C |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 06:24, 24 July 2013
Description
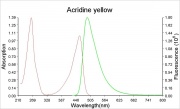
Yellow crystals that produce dark brown solution. Dilute solutions of acridine yellow are fluorescent. Acridine yellow is used as a fluorochrome for biological staining. It has a mean excitation wavelength of 470 nm (blue) and a mean emission wavelength of 550 nm (Wolbers et al., 1990).
Synonyms and Related Terms
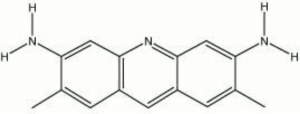
CI 46025; 3,6-diamino-2,7-dimethylacridine hydrochloride
Other Properties
Soluble in ethanol, ether, hydrocarbons, carbon disulfide, boiling water. Insoluble in water, benzene.
Maximum absorption wavelength = 470 nm;
Maximum emission wavelength = 550 nm
| Composition | C13H11N3 |
|---|---|
| CAS | 135-49-9 |
| Melting Point | 281 |
| Molecular Weight | mol. wt. = 273.76 |
Hazards and Safety
Strongly irritating to skin and mucous membranes.
Fisher Scientific: MSDS
Additional Information
R. Wolbers, N. Sterman, C. Stavroudis, Notes for Workshop on New Methods in the Cleaning of Paintings, Getty Conservation Institute, Los Angeles, 1990.
Authority
- Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, Notes for Workshop on New Methods in the Cleaning of Paintings, J.Paul Getty Trust, Los Angeles, 1990
- Website address 1 Comment: Fisher Scientific at: https://www1.fishersci.com/catalogs/acrosgroup.jsp?catalogParamId=8013896&catalogParamType=AG mp = >400C