Difference between revisions of "Sodium phosphate, dibasic"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | White, crystalline, hygroscopic powder. Dibasic sodium phosphate, or DSP, occurs in several hydrated forms from anhydrous to dodecahydrate. It is used in the manufacture of [http://cameo.mfa.org/materials/fullrecord.asp?name=dye dyes], fertilizers, [http://cameo.mfa.org/materials/fullrecord.asp?name=detergent detergents], [http://cameo.mfa.org/materials/fullrecord.asp?name=ceramic ceramics], and [http://cameo.mfa.org/materials/fullrecord.asp?name=enamel | + | White, crystalline, hygroscopic powder. Dibasic sodium phosphate, or DSP, occurs in several hydrated forms from anhydrous to dodecahydrate. It is used in the manufacture of [http://cameo.mfa.org/materials/fullrecord.asp?name=dye dyes], fertilizers, [http://cameo.mfa.org/materials/fullrecord.asp?name=detergent detergents], [http://cameo.mfa.org/materials/fullrecord.asp?name=ceramic ceramics], and [http://cameo.mfa.org/materials/fullrecord.asp?name=enamel%2C%20inorganic enamels]. DSP is used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=sequestrant sequestrant], [http://cameo.mfa.org/materials/fullrecord.asp?name=emulsifier emulsifier], and [http://cameo.mfa.org/materials/fullrecord.asp?name=flame%20retardant fire retardant]. It acts as a [http://cameo.mfa.org/materials/fullrecord.asp?name=mordant mordant] in dyeing and is used for weighting [http://cameo.mfa.org/materials/fullrecord.asp?name=silk silk]. DSP is also used as a replacement for [http://cameo.mfa.org/materials/fullrecord.asp?name=borax borax] in [http://cameo.mfa.org/materials/fullrecord.asp?name=solder soldering] and [http://cameo.mfa.org/materials/fullrecord.asp?name=brazing%20solder brazing]. In addition, DSP is used in [http://cameo.mfa.org/materials/fullrecord.asp?name=bleaching%20agent bleach] baths for color photographs. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 42: | Line 42: | ||
== Authority == | == Authority == | ||
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Thomas C. Jester (ed.), ''Twentieth-Century Building Materials'', McGraw-Hill Companies, Washington DC, 1995 |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8805 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8805 | ||
Revision as of 06:25, 24 July 2013
Description
White, crystalline, hygroscopic powder. Dibasic sodium phosphate, or DSP, occurs in several hydrated forms from anhydrous to dodecahydrate. It is used in the manufacture of dyes, fertilizers, detergents, ceramics, and enamels. DSP is used as a sequestrant, emulsifier, and fire retardant. It acts as a mordant in dyeing and is used for weighting silk. DSP is also used as a replacement for borax in soldering and brazing. In addition, DSP is used in bleach baths for color photographs.
Synonyms and Related Terms
DSP; disodium phosphate; secondary sodium orthophosphate; disodium hydrogen phosphate; phosphate of soda; Sorensen's phosphate (dihydrate)
Other Properties
Soluble in water, ethanol.
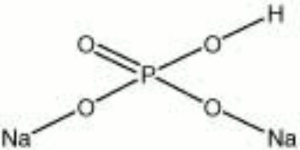
| Composition | Na2HPO4 - xH2O |
|---|---|
| CAS | 7558-79-4 |
| Melting Point | 240 |
| Density | 2.07 |
| Molecular Weight | mol. wt. = 141.96 |
| Refractive Index | 1.4412, 1.4424, 1.4526 |
Hazards and Safety
Nonflammable.
Mallinckrodt Baker: MSDS
Authority
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Thomas C. Jester (ed.), Twentieth-Century Building Materials, McGraw-Hill Companies, Washington DC, 1995
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8805
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index = 1.4412, 1.4424, 1.4526