Difference between revisions of "Tris(hydroxymethyl)aminomethane"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 41: | Line 41: | ||
== Authority == | == Authority == | ||
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, ''Notes for Workshop on New Methods in the Cleaning of Paintings'', J.Paul Getty Trust, Los Angeles, 1990 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 06:25, 24 July 2013
Description
White, crystalline solid. Tris(hydroxymethyl)aminomethane is used as an emulsifying agent for oils, fats and waxes. It is also mixed in equally proportions with its corresponding hydrochloric acid salt and used as a buffer (see Trizma).
Synonyms and Related Terms
tris; tris hydroxymethyl aminomethane; THAM; 2-amino-2-hydroxymethyl-1,3-propanediol; tris amine buffer
Other Properties
Soluble in water (pH = 10.36 for 0.1 M solution).
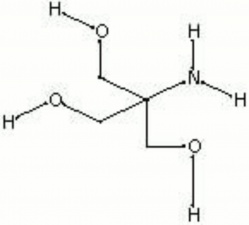
| Composition | (CH2OH)3CNH2 |
|---|---|
| CAS | 77-86-1 |
| Melting Point | 171-172 |
| Molecular Weight | mol. wt. = 121.14 |
| Boiling Point | 219-220 |
Hazards and Safety
Combustible. Corrosive to copper, brass, aluminum.
Harmful by ingestion and inhalation. Skin contact causes irritation and burns.
Mallinckrodt Baker: MSDS
Authority
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, Notes for Workshop on New Methods in the Cleaning of Paintings, J.Paul Getty Trust, Los Angeles, 1990