Difference between revisions of "Mercaptobenzothiazole"
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Pale yellow needles with a disagreeable odor. Mercaptobenzothiazole has been used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=fungicide fungicide] in paper (Roberts and Etherington 1982). It is used industrially as a [http://cameo.mfa.org/materials/fullrecord.asp?name=corrosion | + | Pale yellow needles with a disagreeable odor. Mercaptobenzothiazole has been used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=fungicide fungicide] in paper (Roberts and Etherington 1982). It is used industrially as a [http://cameo.mfa.org/materials/fullrecord.asp?name=corrosion%20inhibitor corrosion inhibitor] in cutting oils and a [http://cameo.mfa.org/materials/fullrecord.asp?name=vulcanization vulcanization] accelerator for [http://cameo.mfa.org/materials/fullrecord.asp?name=rubber%2C%20natural rubber]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 45: | Line 45: | ||
== Authority == | == Authority == | ||
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 06:31, 24 July 2013
Description
Pale yellow needles with a disagreeable odor. Mercaptobenzothiazole has been used as a fungicide in paper (Roberts and Etherington 1982). It is used industrially as a corrosion inhibitor in cutting oils and a vulcanization accelerator for rubber.
Synonyms and Related Terms
mercapto-benzothiazole; MBT; 2-mercaptobenzothiazole; 2(3H)-benzothiazolethione; 2-benzothiazolethiol; Captax; Dermacid; Mertax; Thiotax; Vulkacit Mercapto; benzothiazole-2-thione
Other Properties
Soluble in ethanol; ether; acetone, benzene, glacial acetic acid and dilute alkalis. Slightly soluble in carbon tetrachloride and naphtha. Insoluble in water.
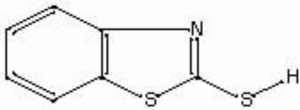
| Composition | C5H4CSC(SH)N |
|---|---|
| CAS | 149-30-4 |
| Melting Point | 180.2-181.7 |
| Density | 1.42 |
| Molecular Weight | mol. wt. = 167.3 |
Hazards and Safety
Toxic by ingestion and inhalation. Contact causes irritation
Combustibe relasing toxic fumes (sulfur and nitrogen oxides).
International Chemical Safety Card
Additional Information
M.Roberts, D.Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington, DC, 1982.
Authority
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982