Difference between revisions of "Neutral red"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A dark green powder that dissolves in [http://cameo.mfa.org/materials/fullrecord.asp?name=water water] or [http://cameo.mfa.org/materials/fullrecord.asp?name=ethyl | + | A dark green powder that dissolves in [http://cameo.mfa.org/materials/fullrecord.asp?name=water water] or [http://cameo.mfa.org/materials/fullrecord.asp?name=ethyl%20alcohol ethanol] to form a red solution. Neutral red is used as an indicator for alkalinity of water and [http://cameo.mfa.org/materials/fullrecord.asp?name=urea urea]. It is red below pH 6.8 and yellow above pH 8.0. It is also used to prepare neutral red indicating paper. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 38: | Line 38: | ||
== Authority == | == Authority == | ||
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6571 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6571 | ||
Revision as of 07:32, 24 July 2013
Description
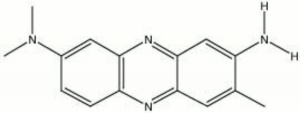
A dark green powder that dissolves in water or ethanol to form a red solution. Neutral red is used as an indicator for alkalinity of water and urea. It is red below pH 6.8 and yellow above pH 8.0. It is also used to prepare neutral red indicating paper.
Synonyms and Related Terms
toluylene red; neutral red chloride; nuclear fast red; Michrome no. 226; 3-amino-7-(dimethylamino)-2-methylphenazine monohydrochloride; Basic Red 5; CI 50040
Other Properties
Soluble in water, ethanol producing a red color. Insoluble in xylene.
Absorption max. = 533 nm
| Composition | C15H17ClN4 |
|---|---|
| CAS | 553-24-2 |
| Melting Point | 290 |
| Molecular Weight | mol. wt. = 288.77 |
Hazards and Safety
Contact may cause irritation.
Fisher Scientific: MSDS
Authority
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6571