Difference between revisions of "Perchloroethylene"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 45: | Line 45: | ||
== Authority == | == Authority == | ||
| − | * | + | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Hoechst Celanese Corporation, ''Dictionary of Fiber & Textile Technology'' (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 06:32, 24 July 2013
Description
Colorless chlorinated hydrocarbon with an ether-like odor. Perchloroethylene is used as a dry-cleaning solvent and as a vapor-degreaser for metals.
Synonyms and Related Terms
tetrachloroethylene; TCE; tetrachloroethene; ethylene tetrachloride; Perclene; Vaclene [DuPont]
Other Properties
Miscible in ethanol, ether, chloroform, benzene. Insoluble in water.
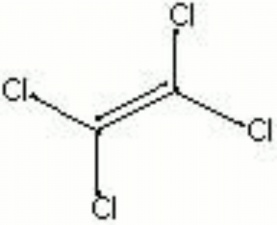
| Composition | Cl2C:CCl2 |
|---|---|
| CAS | 127-18-4 |
| Melting Point | 22 |
| Density | 1.6230 |
| Molecular Weight | mol. wt. = 165.8 |
| Refractive Index | 1.5055 |
| Boiling Point | 121 |
Hazards and Safety
Irritating to eyes and skin. Potential carcinogen. Nonflammable, but may decompose in the presence of flames, hot surfaces or UV light to form toxic and corrosive fumes (hydrogen chloride, phosgene, chlorine). Decomposes slowly on contact with moisture producing trichloroacetic acid and hydrochloric acid.
International Chemical Safety Card
Authority
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990