Difference between revisions of "Lead sulfate"
(username removed) |
(username removed) |
||
| Line 63: | Line 63: | ||
== Authority == | == Authority == | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 444 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5444 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5444 | ||
| Line 71: | Line 71: | ||
* Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Lead_sulfate (Accessed Sept. 7, 2005) | * Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Lead_sulfate (Accessed Sept. 7, 2005) | ||
| − | * | + | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 06:44, 24 July 2013
Description
A white, heavy powder that is used as a pigment. Lead sulfate occurs naturally in the mineral anglesite. It is synthesized by adding sulfuric acid to a lead salt solution. Lead sulfate is used in lithography and in weighting fabrics. It is also used as a paint drier.
Synonyms and Related Terms
lead (II) sulfate; Pigment White 3; CI 77630; anglesite (mineral); lead sulphate (Br.); sulfato de plomo (Esp.); Metallweiss (Deut.); Milchweiss (Deut.); Bleisulfat (Deut.); Anglesit (Deut.); sulfate de plomb (Fr.); theiikos molybdos (Gr.); solfato di piombo (It.); loodsulfaat (Ned.); sulfato de chumbo (Port.); white lead; Flemish white; Mulhaus white; Mulhouse white; milk white
Other Properties
Soluble in sodium hydroxide solution, concentrated hydriodic acid. Slightly solution in hot water. Insoluble in ethanol.
Transparent colorless particles showing high relief and moderate birefringence under crossed polars
| Composition | PbSO4 |
|---|---|
| CAS | 7446-14-2 |
| Mohs Hardness | 2.75 |
| Melting Point | 1170 |
| Density | 6.12-6.39 |
| Molecular Weight | mol. wt. = 303.28 |
| Refractive Index | 1.878; 1.883; 1.895 |
Hazards and Safety
Toxic by inhalation or ingestion. Noncombustible. Skin contact may cause irritation or ulcers. Carcinogen, teratogen, suspected mutagen.
Additional Information
M-C. Corbeil, P.J. Sirois, E.A. Moffatt, "The use of a white pigment patented by Freeman by Tom Thomson and the Group of Seven" preprints ICOM, Lyons, 1999. p.369.
Comparisons
Characteristics of Common White Pigments
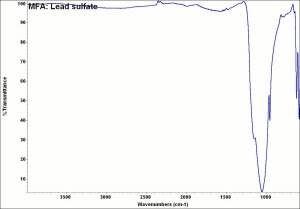
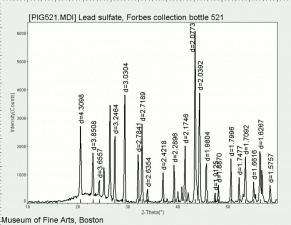
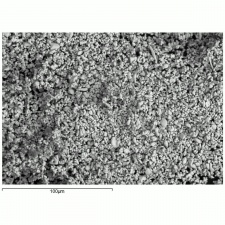
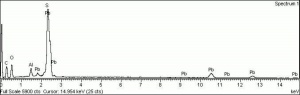
Additional Images
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 444
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5444
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Lead_sulfate (Accessed Sept. 7, 2005)
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985