Difference between revisions of "Brucite"
(username removed) |
(username removed) |
||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A white to gray mineral composed of [http://cameo.mfa.org/materials/fullrecord.asp?name=magnesium | + | A white to gray mineral composed of [http://cameo.mfa.org/materials/fullrecord.asp?name=magnesium%20hydroxide magnesium hydroxide]. Brucite was named for Archibald Bruce, an American mineralogist in the late 18th and early 19th centuries. It occurs naturally in deposits, often with [http://cameo.mfa.org/materials/fullrecord.asp?name=serpentine serpentine] and [http://cameo.mfa.org/materials/fullrecord.asp?name=dolomite dolomite], in Italy (Teulada), Sweden (Jakobsberg, Filipstad, Nordmark), Canada, and the U.S. (Nevada, New Jersey, Pennsylvania, Texas). The soft mineral can be transparent to translucent with a pearly luster. Brucite is used as a refractory material as well as for a source of [http://cameo.mfa.org/materials/fullrecord.asp?name=magnesium magnesium] metal and [http://cameo.mfa.org/materials/fullrecord.asp?name=magnesia magnesia]. |
[[File:pb20804brucite.jpg|thumb|Brucite]] | [[File:pb20804brucite.jpg|thumb|Brucite]] | ||
| Line 39: | Line 39: | ||
== Additional Information == | == Additional Information == | ||
| − | Mineralogy Database: [http://www.webmineral.com/data/Brucite.shtml Brucite] | + | ° Mineralogy Database: [http://www.webmineral.com/data/Brucite.shtml Brucite] |
== Authority == | == Authority == | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "brucite" | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "brucite" Encyclopædia Britannica [Accessed December 4, 2001]. |
| − | * | + | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Brucite (Accessed Sept. 2, 2005) | * Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Brucite (Accessed Sept. 2, 2005) | ||
| Line 53: | Line 53: | ||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* MSDS Sheet Comment: density - 2.36 | * MSDS Sheet Comment: density - 2.36 | ||
Revision as of 06:48, 24 July 2013
Description
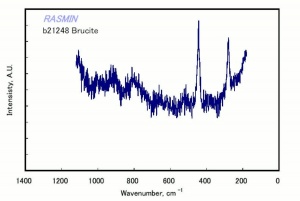
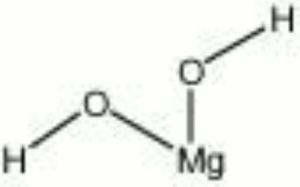
A white to gray mineral composed of magnesium hydroxide. Brucite was named for Archibald Bruce, an American mineralogist in the late 18th and early 19th centuries. It occurs naturally in deposits, often with serpentine and dolomite, in Italy (Teulada), Sweden (Jakobsberg, Filipstad, Nordmark), Canada, and the U.S. (Nevada, New Jersey, Pennsylvania, Texas). The soft mineral can be transparent to translucent with a pearly luster. Brucite is used as a refractory material as well as for a source of magnesium metal and magnesia.
Synonyms and Related Terms
nemalite; magnesium hydroxide; magnesium hydrate; milk of magnesia; brucita (Esp.); brucite (Port.); Brucit (Deut.); bruciet (Ned.)
Other Properties
Tabular, rhombohedron crystals, may sometimes be fibrous. Perfect cleavage parallel to prism base. Luster = waxy to vitreous. Streak = white.
| Composition | Mg(OH)2 |
|---|---|
| CAS | 1309-42-8 |
| Mohs Hardness | 2.5 |
| Density | 2.39 |
| Molecular Weight | mol. wt. = 58.32 |
Hazards and Safety
Mallinckrodt Baker: MSDS
Additional Information
° Mineralogy Database: Brucite
Authority
- Encyclopedia Britannica, http://www.britannica.com Comment: "brucite" Encyclopædia Britannica [Accessed December 4, 2001].
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Brucite (Accessed Sept. 2, 2005)
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- MSDS Sheet Comment: density - 2.36