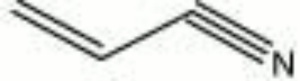Difference between revisions of "Acrylonitrile"
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
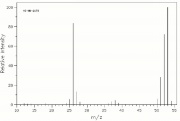
| − | [[File: | + | [[File:acrylonitrilems.jpg|thumb|Mass spectrum of acrylonitrile]] |
== Description == | == Description == | ||
| − | A clear, colorless liquid. Acrylonitrile is the monomer used for many acrylic and modacrylic fibers (see [http://cameo.mfa.org/materials/fullrecord.asp?name=acrylic | + | A clear, colorless liquid. Acrylonitrile is the monomer used for many acrylic and modacrylic fibers (see [http://cameo.mfa.org/materials/fullrecord.asp?name=acrylic%20fiber acrylic fiber]). It is also widely used as a copolymer. Acrylonitrile is copolymerized with butadiene to produce [http://cameo.mfa.org/materials/fullrecord.asp?name=nitrile%20rubber nitrile rubber]. It is an important component in the tough resilient rubber mixtures of ABS ([http://cameo.mfa.org/materials/fullrecord.asp?name=acrylonitrile%20butadiene%20styrene%20resin acrylonitrile-butadiene-styrene]) and SAN ([http://cameo.mfa.org/materials/fullrecord.asp?name=styrene-acrylonitrile styrene-acrylonitrile]). Acrylonitrile is used as an [http://cameo.mfa.org/materials/fullrecord.asp?name=insecticide insecticide]. |
See also [http://cameo.mfa.org/materials/fullrecord.asp?name=polyacrylonitrile polyacrylonitrile]. | See also [http://cameo.mfa.org/materials/fullrecord.asp?name=polyacrylonitrile polyacrylonitrile]. | ||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 13: | Line 12: | ||
Examples: Acritet; Fumigrain; Ventox; | Examples: Acritet; Fumigrain; Ventox; | ||
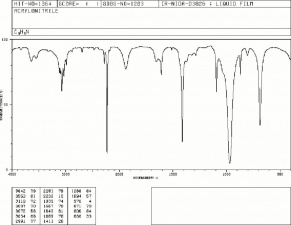
| − | [[[SliderGallery rightalign|acrylonitrile.jpg~Chemical structure]]] | + | [[[SliderGallery rightalign|acrylonitrileir.jpg~FTIR|acrylonitrile.jpg~Chemical structure]]] |
== Other Properties == | == Other Properties == | ||
| Line 51: | Line 50: | ||
== Authority == | == Authority == | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 11 |
| − | * | + | * Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 |
| − | * | + | * Hoechst Celanese Corporation, ''Dictionary of Fiber & Textile Technology'' (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 133 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 133 | ||
Revision as of 06:52, 24 July 2013
Description
A clear, colorless liquid. Acrylonitrile is the monomer used for many acrylic and modacrylic fibers (see acrylic fiber). It is also widely used as a copolymer. Acrylonitrile is copolymerized with butadiene to produce nitrile rubber. It is an important component in the tough resilient rubber mixtures of ABS (acrylonitrile-butadiene-styrene) and SAN (styrene-acrylonitrile). Acrylonitrile is used as an insecticide.
See also polyacrylonitrile.
Synonyms and Related Terms
acrilonitrilo (Esp.); cianoetileno (Esp.); 2-propenonitrilo (Esp.); acrylonitrile (Fr.); acrilonitrile (It.); acrilonitrilo (Port.); vinyl cyanide; 2-propenenitrile; cyanoethylene;
Examples: Acritet; Fumigrain; Ventox;
Other Properties
Soluble in all common organic solvents. Miscible with water.
| Composition | CH2:CHCN |
|---|---|
| CAS | 107-13-1 |
| Melting Point | -83.55 |
| Density | 0.8060 |
| Molecular Weight | mol. wt. = 53.06 |
| Refractive Index | 1.3888 |
| Boiling Point | 77.3 |
Hazards and Safety
Explosive and flammable. Toxic by inhalation and skin absorption. Suspected carcinogen.
International Chemical Safety Card
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 11
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 133