Difference between revisions of "Thymol blue"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 36: | Line 36: | ||
== Authority == | == Authority == | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 184 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9541 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9541 | ||
Revision as of 06:56, 24 July 2013
Description
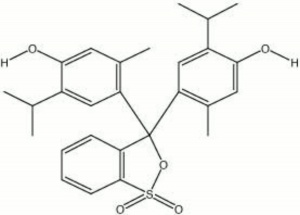
Brownish crystals. Thymol blue dissolves in alkaline solution to form a deep blue color. It acts as an acid base indicator for pH range 1.2-2.8 changing from red to yellow and for pH range 8.0-9.6 changing from red to blue.
Synonyms and Related Terms
Thymolblau (Deut.); thymolsulfonphthalein
Other Properties
Soluble in ethanol, dilute alkali. Insoluble in water. pH: below 1.5 (red); 2.8-8.0 (yellow); 9.6 and above (blue).
| Composition | C27H30O4 |
|---|---|
| CAS | 76-61-9 |
| Melting Point | 223 (dec) |
| Molecular Weight | mol. wt. = 466.60 |
Hazards and Safety
Contact may cause irritation.
Mallinckrodt Baker: MSDS
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 184
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9541