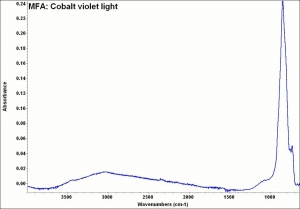
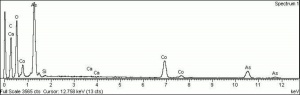
Difference between revisions of "Cobalt violet, light"
| Line 35: | Line 35: | ||
== Additional Information == | == Additional Information == | ||
| − | ° Pigments Through the Ages: [http://webexhibits.org/pigments/indiv/overview/coviolet.html Cobalt violet]° Corbeil, Marie-Claude, Jean-Pierre Charland, Elizabeth Moffatt. 'The characterization of cobalt violet pigments' ''Studies in Conservation'' vol.47 (2002), pp.237-249. | + | ° Pigments Through the Ages: [http://webexhibits.org/pigments/indiv/overview/coviolet.html Cobalt violet] |
| + | |||
| + | ° Corbeil, Marie-Claude, Jean-Pierre Charland, Elizabeth Moffatt. 'The characterization of cobalt violet pigments' ''Studies in Conservation'' vol.47 (2002), pp.237-249. | ||
== Authority == | == Authority == | ||
Revision as of 10:43, 13 January 2014
Description
A pale to medium violet pigment originally composed of Cobaltous arsenate. Cobaltous arsenate occurs in nature as cobalt bloom or Erythrite. Once it was synthetically produced in 1880, it became an important permanent, violet pigment. Cobaltous arsenate is now rarely used because of its toxicity. It has been replaced by the use of Cobaltous phosphate (deep cobalt violet) and Cobaltous ammonium phosphate. Light cobalt violet was used as a colorant in paints, glass, glazes, and enamels.
Synonyms and Related Terms
cobalt arsenate (arsenite); Pigment Violet 14; CI 77350; erythrite (mineral); violeta de cobalto claro (Esp.); Kobaltviolett (hell) (Deut.); violet de cobalt (clair) (Fr.); iodes toy kobaltioy anoikto (Gr.); violetto di cobalto chiaro (It.); cobalt violet (licht) (Ned.); violeta de cobalto, claro (Port.); red cobalt; cobalt bloom; ; pale cobalt violet
Other Properties
Monoclinic crystal system with prismatic or euhedral crystals. Perfect cleavage parallel to long axes. Weakly pleochroic. High birefringence under crossed polars.
| Composition | Co3(AsO4)2 - 8H2O |
|---|---|
| Mohs Hardness | 1.5-2.5 (erythrite) |
| Density | 3.06 |
| Refractive Index | 1.626-1.701 |
Hazards and Safety
Highly toxic by ingestion, inhalation, and contact.
Additional Information
° Pigments Through the Ages: Cobalt violet
° Corbeil, Marie-Claude, Jean-Pierre Charland, Elizabeth Moffatt. 'The characterization of cobalt violet pigments' Studies in Conservation vol.47 (2002), pp.237-249.
Authority
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000