Difference between revisions of "Acetal"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 43: | Line 43: | ||
Fisher Scientific: [http://www.fishersci.ca/msds2.nsf/0/6617ED9ADBB1F1B485256CA40083A6D4/$file/MSDS-69052.html?open MSDS] | Fisher Scientific: [http://www.fishersci.ca/msds2.nsf/0/6617ED9ADBB1F1B485256CA40083A6D4/$file/MSDS-69052.html?open MSDS] | ||
| − | == | + | == Sources Checked for Data in Record == |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
Revision as of 12:51, 29 April 2016
Description
A colorless, volatile liquid obtained from the reaction of Acetaldehyde and Ethyl alcohol. Acetal has a pleasing odor and a nutlike aftertaste. It is used as a solvent, and as an ingredient in cosmetics, perfumes (such as jasmine) and flavorings.
Synonyms and Related Terms
diethylacetal; 1,1-diethoxyethane; ethylidenediethyl ether; acetaldehyde diethyl acetal
Other Properties
Soluble in hexane, ethanol and ethyl acetate. Slightly soluble in water.
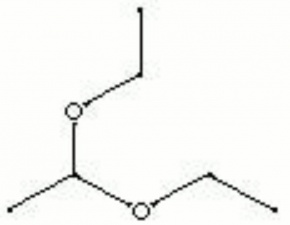
| Composition | CH3CH(OC2H5)2 |
|---|---|
| CAS | 105-57-7 |
| Melting Point | -100 |
| Density | 0.8254 |
| Molecular Weight | mol. wt. = 118.18 |
| Refractive Index | 1.379-1.385 |
| Boiling Point | 102.7 |
Hazards and Safety
Highly flammable. Flash point = -12C. Sensitive to light, moisture and heat. May form explosive peroxides. Contact causes irritation. Narcotic in high concentrations.
Fisher Scientific: MSDS
Sources Checked for Data in Record
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Website address 1 Comment: Fisher Scientific at https://www1.fishersci.com/catalogs/acrosgroup.jsp?catalogParamId=8013176&catalogParamType=AG Flash point = -21C