Difference between revisions of "Fluorescein"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 37: | Line 37: | ||
Mallinckrodt Baker: [http://www.jtbaker.com/msds/englishhtml/f3042.htm MSDS] | Mallinckrodt Baker: [http://www.jtbaker.com/msds/englishhtml/f3042.htm MSDS] | ||
| − | == | + | == Sources Checked for Data in Record == |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Revision as of 20:41, 30 April 2016
Description
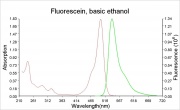
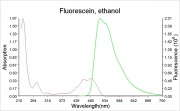
A bright, orange to red crystalline powder that reacts with alkalis to form an intense green Fluorescence. Fluorescein is used for the detection of soluble hydroxides, carbonates, and ammonia. It is sensitive to the part per billion level. Its UV absorption maxima occur at 493.4 and 460 nm. Fluorescein is used as an indicator and as a textile dye. In the late 19th century, fluorescein was used as a yellow dye on Wool and Silk without a mordant.
Synonyms and Related Terms
Solvent Yellow 94; CI 45350:1; D&C Yellow No.7; 3',6'-dihydroxyspiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one; resorcinolphthalein; Fluorescein (Deut.); fluorescéïne (Fr.); diresorcinolphthalein;
Other Properties
Soluble in hot ethanol, glacial acetic acid. Soluble in alkalis and ammonia producing a bright green fluorescence. Insoluble in water, benzene, chloroform, ether.
The fluorescence of fluorescein changes with pH (colorless below pH=4.0 and fluorescent green above 4.5)
| Composition | C20H12O5 |
|---|---|
| CAS | 2321-07-2 |
| Melting Point | 314-316 |
| Molecular Weight | mol. wt. = 332.32 |
Hazards and Safety
Combustible.
Mallinckrodt Baker: MSDS
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry # 4194
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: The fluorescence of fluorescein changes with pH. It is colorless below pH=4.0 and above 4.5, it fluoresces green.
- Sigma Dyes, Stains and Natural Pigments, Infrared Library, Nicolet, 1991-1995
- F. Crace-Calvert, Dyeing and Calico Printing, Palmer & Howe, London, 1876 Comment: p. 251