Difference between revisions of "Hydroxyacetic acid"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 37: | Line 37: | ||
Mallinckrodt Baker: [http://www.jtbaker.com/msds/englishhtml/g6820.htm MSDS] | Mallinckrodt Baker: [http://www.jtbaker.com/msds/englishhtml/g6820.htm MSDS] | ||
| − | == | + | == Sources Checked for Data in Record == |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 | ||
Revision as of 05:28, 1 May 2016
Description
Colorless, deliquescent crystals that occur naturally as a component in sugarcane. Hydroxyacetic acid, or glycolic acid, is a weak acid. It is sold commercially as a 70% solution. It is used in processing and dyeing textiles and Leather. Hydroxyacetic acid is also used for cleaning, polishing, and soldering metals.
Synonyms and Related Terms
glycolic acid; hydroxyethanoic acid
Other Properties
Soluble in water, methanol, ethanol, acetone, acetic acid, ether.
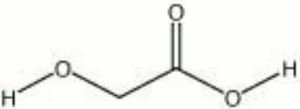
| Composition | CH2OHCOOH |
|---|---|
| CAS | 79-14-1 |
| Melting Point | 80 |
| Density | 1.27 |
| Molecular Weight | mol. wt. = 76.05 |
Hazards and Safety
Corrosive. Contact causes irritation and burns.
Mallinckrodt Baker: MSDS
Sources Checked for Data in Record
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4508