Difference between revisions of "Malic acid"
Jump to navigation
Jump to search
(username removed) |
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
||
| Line 39: | Line 39: | ||
Mallinckrodt Baker: [http://www.jtbaker.com/msds/englishhtml/m0403.htm MSDS] | Mallinckrodt Baker: [http://www.jtbaker.com/msds/englishhtml/m0403.htm MSDS] | ||
| − | == | + | == Sources Checked for Data in Record == |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 55 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 55 | ||
Revision as of 06:50, 1 May 2016
Description
White, water-soluble crystals that occur naturally in apples and other fruits. Malic acid is used as a chelating and buffering agent. The weak acid is also used to acidify food and to age wine.
Synonyms and Related Terms
apple acid; hydroxysuccinic acid; hydroxybutanedioic acid
Other Properties
Soluble in water, methanol, diethyl ether, acetone and ethanol. Slightly soluble in ether. Insoluble in benzene.
pH = 2.2 (0.1 N solution)
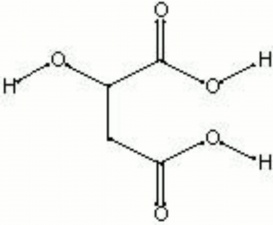
| Composition | COOHCH2CH(OH)COOH |
|---|---|
| CAS | 617-48-1 (dl form) |
| Melting Point | 131-132 |
| Density | 1.601 |
| Molecular Weight | mol. wt. = 134.09 |
Hazards and Safety
Combustible. Contact may cause irritation.
Mallinckrodt Baker: MSDS
Sources Checked for Data in Record
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 55
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5747
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: pH = 2.2 (0.1 N solution)