Difference between revisions of "Molybdenum trioxide"
Jump to navigation
Jump to search
(username removed) |
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
||
| Line 40: | Line 40: | ||
Mallinckrodt Baker: [http://www.jtbaker.com/msds/englishhtml/m7829.htm MSDS] | Mallinckrodt Baker: [http://www.jtbaker.com/msds/englishhtml/m7829.htm MSDS] | ||
| − | == | + | == Sources Checked for Data in Record == |
* ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 6321 | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 6321 | ||
Revision as of 13:59, 1 May 2016
Description
A white powder that melts to a yellow liquid then solidifies to form pale yellow crystals. Molybdenum trioxide is used as a pigment in ceramic glazes and enamels.
Synonyms and Related Terms
molybdenum anhydride; molybdic oxide; molybdic acid hydride
Other Properties
Soluble in concentrated mineral acids and alkalis. Slightly soluble in water.
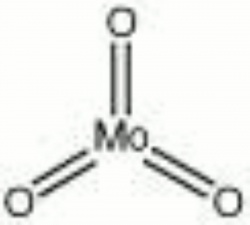
| Composition | MoO3 |
|---|---|
| CAS | 1313-27-5 |
| Melting Point | 795 |
| Density | 4.69 |
| Molecular Weight | mol. wt. = 143.94 |
| Boiling Point | 1150 |
Hazards and Safety
Toxic by inhalation and ingestion. Contact causes irritation.
Mallinckrodt Baker: MSDS
Sources Checked for Data in Record
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 6321
- Robert Fournier, Illustrated Dictionary of Practical Pottery, Chilton Book Company, Radnor, PA, 1992
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993