Difference between revisions of "Titanium tetrachloride"
Jump to navigation
Jump to search
(username removed) |
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
||
| Line 40: | Line 40: | ||
LINK: [http://www.cdc.gov/niosh/ipcsneng/neng0022.html International Chemical Safety Card] | LINK: [http://www.cdc.gov/niosh/ipcsneng/neng0022.html International Chemical Safety Card] | ||
| − | == | + | == Sources Checked for Data in Record == |
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
Revision as of 22:20, 1 May 2016
Description
Colorless, fuming liquid that forms dense white cloud in moist air. Titanium tetrachloride is used to make iridescent glass and artificial pearls. It was formerly used with potassium bitartrate as a mordant for dyeing textiles and leather.
Synonyms and Related Terms
titanium chloride; titanic chloride; tetrachlorotitanium
Other Properties
Soluble in water, dilute hydrochloric acid.
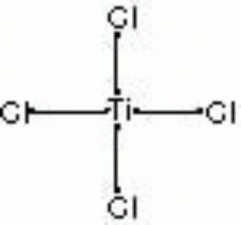
| Composition | TiCl4 |
|---|---|
| CAS | 7550-45-0 |
| Melting Point | -24.1 |
| Density | 1.726-1.761 |
| Molecular Weight | mol. wt. = 189.7 |
| Boiling Point | 136.4 |
Hazards and Safety
Toxic by inhalation. Corrosive. Contact causes irritation and burns.
LINK: International Chemical Safety Card
Sources Checked for Data in Record
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 9618
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993