Difference between revisions of "Tris(hydroxymethyl)aminomethane"
Jump to navigation
Jump to search
(username removed) |
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
||
| Line 39: | Line 39: | ||
Mallinckrodt Baker: [http://www.jtbaker.com/msds/englishhtml/t7111.htm MSDS] | Mallinckrodt Baker: [http://www.jtbaker.com/msds/englishhtml/t7111.htm MSDS] | ||
| − | == | + | == Sources Checked for Data in Record == |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Revision as of 21:28, 1 May 2016
Description
White, crystalline solid. Tris(hydroxymethyl)aminomethane is used as an emulsifying agent for oils, fats and waxes. It is also mixed in equally proportions with its corresponding hydrochloric acid salt and used as a buffer (see Trizma).
Synonyms and Related Terms
tris; tris hydroxymethyl aminomethane; THAM; 2-amino-2-hydroxymethyl-1,3-propanediol; tris amine buffer
Other Properties
Soluble in water (pH = 10.36 for 0.1 M solution).
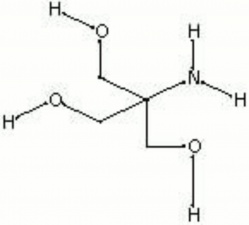
| Composition | (CH2OH)3CNH2 |
|---|---|
| CAS | 77-86-1 |
| Melting Point | 171-172 |
| Molecular Weight | mol. wt. = 121.14 |
| Boiling Point | 219-220 |
Hazards and Safety
Combustible. Corrosive to copper, brass, aluminum.
Harmful by ingestion and inhalation. Skin contact causes irritation and burns.
Mallinckrodt Baker: MSDS
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, Notes for Workshop on New Methods in the Cleaning of Paintings, J.Paul Getty Trust, Los Angeles, 1990